This essay is the first of a planned three-part series delving into the science of ‘first and second nature.’
“Man did not create society; society existed before Man.”
Peter Kropotkin, “The State: Its Historic Role”
“Unless we understand the language of the fauna and flora, we will neither understand ourselves nor become ecological socialists.”
Abdullah Öcalan, The Age of Masked Gods and Disguised Kings
At the center of many critiques lobbed at social ecology from other currents of radical environmental thought lies its humanism and alleged anthropocentrism. Murray Bookchin’s philosophical project resituated humanity within the long arc of natural history and within the present webs of ecological interdependence and entanglement, but nevertheless retained a special place for us in that view of nature—much to the consternation of deep ecologists who saw this as an insufficient break with human supremacist thinking and the western mythology of the Great Chain of Being. These debates—held in environmental movement congresses, frontline direct actions, and the pages of academic discourse’s radical fringes—have operated primarily at the level of the philosophical. Ecological philosophy will of course remain central in this essay, but my own engagement with prevailing social ecology dogma on the place of human beings in natural history will instead focus on the science. Our more recent leaps in scientific understanding of some of these questions necessitate, in my view, substantial philosophical revisions—particularly to the concept of second nature.
Both social ecology and deep ecology see human beings as a part of nature, but in importantly different ways. For deep ecologists, all living beings bear inherent value, while collectively making up an ecological totality to which they are subject. Humans and hummingbirds and bacteria alike are beholden to Nature’s laws of carrying capacity and balance. It is an ecological philosophy of humility. Social ecologists, by contrast, see human societies as notably distinct from all the other ways living beings inhabit our magnificent world: firstly, because our ways of organizing ourselves socially are so endlessly (and consciously) mutable, and secondly and most critically, because how we as one particular species relate to each other shapes the future of all other life on Earth. The ecological crisis is, social ecologists maintain, a (human) social crisis, stemming from our (not hummingbirds’ or bacteria’s) social relations. Our protean sociality and the “power to create and the power to destroy” that comes with it give us a unique responsibility among that tapestry of species that cannot be laid at the feet of any other, no matter how awe-inspiring they may otherwise be.1
Both deep ecology’s decentering and social ecology’s recentering of humanity stem from certain political fears. The former fears flattering the ego of a narcissistic humanity unraveling the world; the latter fears that casting ourselves as “just another species” loses sight of human society’s ability to change, to play a different ecological role. Naturalizing humanity’s present social relations, social ecologists note, is the counter-revolutionary rhetorical move of status quo conservatives and doomer “environmental” misanthropes alike, and must be resolutely resisted. What I will show, however, is that this pair of well-justified political fears sit in a largely unnecessary antagonism, for humanity’s extraordinary capacity for social change is not a lone island in a sea of animalian biological determinism.
For an attempt at developing a comprehensive philosophy of humanity’s relationship to nature, social ecology has surprisingly little to say about the animal question. There have been few engagements by social ecologists with animal liberationist or post-humanist ideas seeking to broaden conceptions of subjectivity, moral worth, and philosophical import to non-humans.2 While Bookchin occasionally alluded to the ethical value of treating animals humanely, his works also paint a rather bleak and limited view of them. When he invokes non-human animals, it is with rare exception to denigrate their complexity, sociality, and intelligence, to elevate human beings to a unique status by contrast. “The ‘ontological divide’ between the nonhuman and the human,” he writes, “is very real.”3 For Bookchin, animals can hardly be called “social” at all, their clustering together in groups being ephemeral, unstructured, and purely instinctive, geared towards particular passing needs of collective predator defense or mating. These loose, not-yet-social forms he terms mere “communities.” “Humans, by contrast…” he writes, “form not only communities, but a new phenomenon called societies.”4
Bookchin used the language of “first nature” and “second nature” to communicate the relationship between nature and human society. He drew these words from Cicero, who wrote “[B]y the use of our hands, we bring into being within the realm of Nature, a second nature for ourselves.” First nature is the process of biological evolution, characterized by a set of developmental tendencies towards differentiation, interdependence, and subjectivity.5 Second nature is made possible by the creative unfolding of first nature, but it cannot adequately be described in biological, Darwinian terms. It is what we might refer to as “society” or “culture,” whose history is not of selective pressures applied to genes but rather is of the dynamics of social development.6
I believe the concepts of first and second nature are philosophically useful and important, but I intend to push them beyond their originally imagined limits and reframe and complexify these ideas in accordance with the present scope of human scientific knowledge. This will be the first in a trilogy of essays on this broad subject. In this piece I will make the case that what social ecologists understand as second nature in fact characterizes many of the behaviors and social forms of a variety of other animal species, requiring a new understanding of second nature that is beyond the human. This has significant implications for the philosophical project of social ecology, and possibly, more speculatively, for its political project.
My primary purpose here is greater clarity within the thought of social ecology, not an attempt at a bridging synthesis between deep and social ecology. But I do think that bringing scientific rigor to the philosophy of social ecology will result in shedding some of its anthropocentric assumptions that presently grant deep ecologists cover to dismiss social ecology’s essential conceptual insights out of hand. Perhaps wedging the door open just a bit wider to productive dialogue between these two radical ecological traditions can be a secondary outcome of my discussion here.
Nature First and Second in Bookchin’s Work
Bookchin is a dialectician, thinking in terms of historical processes rather than static essences. For him, first and second nature are nested evolutionary developments without a single identifiable line of bifurcation between them. It can therefore be a challenge to hone in on what precisely Bookchin means by “second nature.” He describes it as the emergence of human beings out of biological evolution, denoted by a number of important features (symbolic language, adapting their environment, abstract thought, altruistic behavior, etc). If one is to ask a different question (“Might certain nonhuman animals be shaped by or possess a second nature?”), we must construct a more general definition, requiring a more developed theory of the inflection point between the social and the biological.7
In “What Is Social Ecology?” Bookchin states that “Second nature is the way in which human beings…inhabit and alter the natural world.”8 This, however, is not an especially clear distinction between human beings and the rest of life, given that living things across all kingdoms do exactly this. The greatest alterations to the world we inhabit have not been carried out by humans, but by the more humble likes of cyanobacteria, white rot fungus, and ants. Even the more specific matter of niche construction, a phenomenon exemplified by organisms as varied as bison and diatoms, is simply not a viable basis for such a definition. Bookchin immediately acknowledges this, but maintains his assertion on the grounds that the environmental changes caused by humans are “profoundly different” than those by any other living thing, because they entail “considerable technical foresight,” innovation, and social labor. This echoes Karl Marx’s words in Capital: “A spider conducts operations that resemble those of a weaver, and a bee puts to shame many an architect in the construction of her cells. But what distinguishes the worst architect from the best of bees is this, that the architect raises his structure in imagination before he erects it in reality.”9
Whether “technical foresight” is unique to humans among animals is a remaining scientific question (and one for which plenty of specialists firmly disagree with Bookchin and Marx—innovation and social labor at least are most certainly not uniquely human).10 Even if we take as a given that these are unique human faculties, however, this is simply a list of cognitive attributes and behaviors. We have yet to hone in on defining the historically unfolding processes of cumulative change that are second nature.
Fortunately, Bookchin elaborates. He also says (in the same essay),
Nonhuman beings generally live in ecological niches, their behavior guided primarily by instinctive drives and conditioned reflexes. Human societies are “bonded” together by institutions that change radically over centuries. Nonhuman communities are notable for their general fixity, by their clearly preset, often genetically imprinted rhythms. Human communities are guided in part by ideological factors and are subject to changes conditioned by those factors. Nonhuman communities are generally tied together by genetically rooted instinctive factors – to the extent that these communities exist at all.11
We are here getting to the root of the matter: the attributes of second nature are determined by something other than biology, at least in the narrow sense. His presumption of an “instinctive” and “genetically imprinted” stasis of all animal social forms and behaviors is a helpful explanatory foil for the meaning of second nature but is, however, defied by deep observation and research on a number of nonhuman species, which we will discuss.
In a 1996 lecture, Bookchin zeroed in with even more precision and clarity on this understanding of second nature as development that is no longer confined by or taking place within the genome. He states,
We have also evolved in a way that has opened a new area of evolution: a second nature. This new area of evolution is socio-cultural… A new realm of development that is not strictly biological and in fact whose essence is to become less and less biological (which does not mean we can ever escape from our biology). This is the realm of social relations… [T]he most striking feature of human development is precisely the fact that human beings develop beyond their genetic apparatus. That is to say, acquire cultural attributes not just genetic attributes, not just biochemical attributes, that determine their behavior, or that profoundly affect their behavior.12
He describes this historical elaboration (of social relations and cultural practices) as “fundamentally different to the kind you see in the natural world.”
Most succinctly, he writes in Remaking Society that “Although nonhuman animals may approximate human forms of association in many ways, they do not create a ‘second nature’ that embodies a cultural tradition” (emphasis mine).13
While interwoven with a set of empirical claims that I believe are partially or wholly untrue for a wide array of other animal species, Bookchin’s passages here provide the basis of a clear and well-defined distinction between first nature and second nature and point towards some of the comparative standards we would need to hold up to various animal societies to assess the extent to which a second nature may have emerged. In my assessment, the most straightforward and scientifically useful definition of “second nature” is the process of historical change not by natural selection acting upon genetic transmission, but by social processes: social learning, acculturation, and cumulative modification of learned practices and ideas. Such a process of social evolution is dialectically emergent from biological evolution, but possesses dynamics of its own that drive historical changes beyond and unexplainable simply by Darwinian natural selection. In other words, first nature is a process of historic diversification transmitted via one genome into another; second nature is a process of historic diversification transmitted via one mind to another.
In Remaking Society, Bookchin writes,
[S]ociety itself in its most primal form stems very much from nature. Every social evolution, in fact, is virtually an extension of natural evolution into a distinctly human realm… To emphasize that “second nature”…emerges from within primeval “first nature” is to re-establish the fact that social life always has a naturalistic dimension, however much society is pitted against nature in our thinking. Social ecology clearly expresses the fact that society is not a sudden “eruption” in the world. Social life does not necessarily face nature as a combatant in an unrelenting war. The emergence of society is a natural fact that has its origins in the biology of human socialization.14
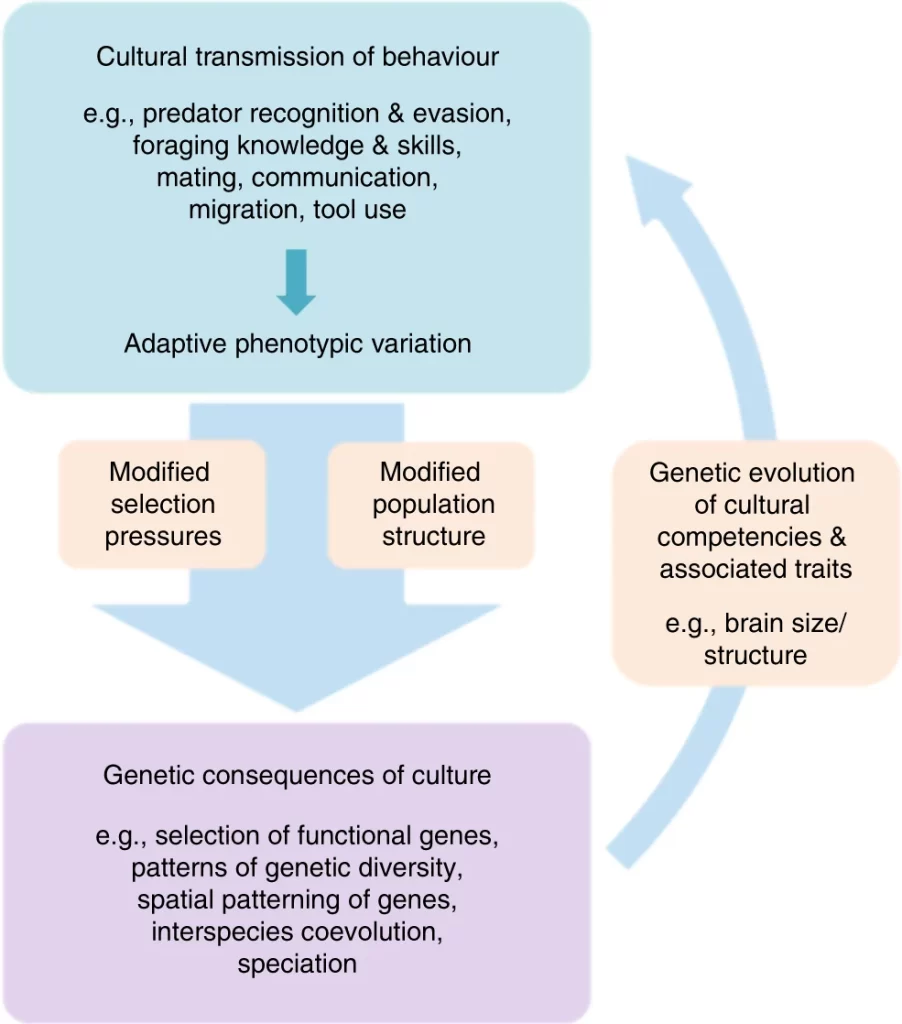
There is a rich scientific literature on these very ideas: that there emerge capacities from our underlying biology for adaptation through social learning, to an extent that is essential to human survival. Indeed, in recent decades it has been recognized how significant a force in biological evolution is social learning and cultural transmission.
Biologists Peter Richerson and Robert Boyd argue that capacity for culture is an evolved trait that enhances biological fitness precisely because of the doors it opens for new adaptive behaviors. They write,
Culture is interesting and important because its evolutionary behavior is distinctly different from that of genes. For example, we will argue that the human cultural system arose as an adaptation, because it can evolve fancy adaptations to changing environments rather more swiftly than is possible by genes alone.15
In his listing of the four contexts in which the modern synthesis (which thinks of evolution narrowly in terms of natural selection acting upon DNA) is inadequate or false, the famed evolutionary biologist John Maynard Smith describes this “cultural inheritance” as “the most important modification” of our ideas about evolution. He writes, “If an animal learns where the water-holes are, or what plants are safe to eat, this information may be transmitted to its offspring, and to more distant descendants. In our own species, cultural inheritance is the basis of historical change.”16
To guide our discussion of how such cultural development may also be rooted in the biology of a number of other animals, I will provide an overview of some key scientific concepts and research, which ought to shape and clarify how we conceive of second nature. These are dual inheritance theory, its related concept of gene-culture coevolution, and social learning, whose mechanisms, research methods, and evidence are fundamental to the study of culture in nonhuman animals. From there, we will turn to case studies of three highly intelligent, highly social mammal species that clearly possess and transmit culture and demonstrate social characteristics that we may arguably consider to be their second nature: African elephants, killer whales, and sperm whales. I wish to be clear from the outset that what I have the space here to discuss is merely the tip of the iceberg. While this small group of charismatic creatures most unambiguously bring to life their own second natures, cultural attributes are far more widespread. In a comprehensive survey, we would be discussing at length not only large-brained mammals but also birds, fish, and even insects. I will attempt to indicate at least glimpses of this diversity through my review of social learning research. Following those three main case studies, I will then conclude with a discussion of why this science is so important: its significance for understanding biodiversity and practicing conservation as part of a revolutionary-ecological political program, and its profound ramifications for social ecology’s ecological philosophy.
Not Just in Our Genes
Human beings are equipped with a variety of evolved characteristics that help them survive in a challenging environment such as, e.g., the Australian interior: legs and feet specialized for energy-efficient long-distance walking, deft hands for tools needed to access a range of food sources, a magnificently adaptive learning cognition to navigate a complex ecology. These remarkable genetic inheritances, however, are necessary but insufficient conditions for the persistence of human communities in that environment for at least sixty thousand years. The extraordinary longevity of these Aboriginal nations is dependent upon a very different sort of inheritance from their ancestors: their transgenerationally accumulated ecological knowledge of plants and animals, their techniques of finding water, their practices of burning landscapes, and their ideological systems of responsibilities towards the many nonhuman inhabitants of the land. None of these things can be found in Aboriginal Australian DNA—their survival over tens of thousands of years has relied heavily upon their cultural inheritance.
This is the principle behind “dual inheritance theory.” We all receive a dual inheritance from our parents: the transmission of genetic information from them and the transmission of cultural information from them. Many of the key evolutionary theorists of culture took as a given that this was a property unique to human beings. In 1977, biologist and Nobel Laureate Peter Medawar wrote that,
Human beings owe their biological supremacy to the possession of a form of inheritance quite unlike that of other animals: exogenetic or exosomatic heredity. In this form of heredity information is transmitted from one generation to the next through non-genetic channels—by word of mouth, by example, and by other forms of indoctrination; in general, by the entire apparatus of culture.17
He was of course correct that this “exogenetic heredity,” as he calls it, is an adaptive breakthrough. But this view of dual inheritance as limited to a single species has collapsed in more recent decades. While pioneered for the study of human beings, these concepts are essential for understanding the role of culture in a variety of animals.
Both sides of this dual inheritance—our genetics and our culture—significantly condition our behavior and reproductive fitness. As primatologist Andrew Whiten writes,
The revelation that cultural inheritance permeates many species’ lives is increasingly recognized to have profound implications for evolutionary biology at large, because it provides a second form of inheritance that builds on the primary genetic inheritance system, facilitating cultural evolution. The two inheritance systems may generate rich interactive effects, as they have in humans.18
In his 1976 book The Selfish Gene, Richard Dawkins attempted to sketch a neo-Darwinian theory of cultural evolution, which imagined discrete packets or units of cultural transmission (such as a single idea or technique for doing something), which he called a “meme.” (It was only much later that this word took on its current online significance.) These memes, in his memetic theory of cultural evolution, are the analogs to genes in biological evolution: those with successful memetic traits will pass theirs along to their children, while less successful memes will fade out of the population.19
Though intriguing, Dawkins’ attempt to explain cultural evolution by way of these Darwinian principles is an overly simplistic one, for two key reasons. First, and most obviously: while genetic inheritance flows only vertically, from parents to offspring, cultural traits are not necessarily transmitted in this way.20 Socially learned traits can be passed on along multiple pathways. Geneticist Luigi Cavalli-Sforza and mathematician-turned-theoretical biologist Marcus Feldman developed mathematical models of the dynamics of cultural evolution when learned behaviors are transmitted vertically, when they are transmitted horizontally (among members of the same generation), and when they are transmitted obliquely (when younger individuals receive cultural instruction from those of an older generation who are not their parents).21 Each of these transmission pathways have radically different outcomes for how a given behavior spreads in a population.22 Kinship relationships are important for cultural transmission patterns, but unlike genes, cultural traits are vastly more flexible and dynamic in their possible spread. They are therefore not directly tied to reproductive outcomes.
The second problem with Darwinian accounts of cultural change is that the emergence of new cultural traits is also much more flexible and dynamic than the genetic equivalent. Genetic innovations, what we call mutations, are chance occurrences. Most of them do not improve biological fitness. Ones that accumulate only do so through selective pressures operating on time scales of many generations. Cultural innovations, however, are often not accidents. Even when they are entirely experimental, they are still the products of thinking minds capable of considering multiple courses of action to solve problems in a complex and dynamic environment. These two factors—the flexibility and dynamism of how new learned practices emerge and the flexibility and dynamism with which they spread—allow cultural evolution to progress far more rapidly and with quite different and more complex dynamics than biological evolution by natural selection. To the extent that culture shapes behavior, it unleashes an entirely new process of historical change.
These distinct processes also interact with one another in fascinating ways. A number of evolutionary theorists have taken to instead talking about this not as “dual inheritance” but as “gene-culture coevolution.”
Gene-culture coevolution is best explained with some human examples. The most famous of these is the evolution of lactose tolerance. When early pastoralists domesticated cattle, sheep, and goats, they began consuming milk and milk products. This was a cultural practice, not a biologically evolved one. Humans, like all mammals, stop producing lactase, the enzyme capable of digesting lactose, after infancy—or at least did until the cultural innovation of animal domestication. Under these new selective pressures generated by a novel cultural practice, groups of people who were raising animals underwent evolutionary genetic changes as well. Genes that extended production of lactase into adulthood predominated, further enabling diets revolving around milk products. This is a coevolution of cultural and genetic traits. Evolution is thereby trailing the agentive or innovative behavior of animals with open-ended decision-making intelligence—tracking closely with what Bookchin terms “participatory evolution.”23
Looking even further back into human evolutionary history, the most important such coevolution is the relationship between hominid mastery of fire and the evolutionary development of our digestive system and much else about our bodies. Fire dramatically expanded the range of foods that could be safely and efficiently digested. It can be thought of as a means of predigestion outside the body, breaking down carbohydrate structures into simpler forms. With a diet of only raw food, a chimpanzee’s gut is nearly three times the size of ours. Relative to chimpanzees, humans require far less food per day and expend far less energy extracting nutrition from it, due to our culturally transmitted trait of cooking food. The physiological changes that have resulted from this also go far beyond the substantially smaller gut. Hominid teeth became smaller with less powerful jaws, and our nutritional efficiency and diversity of potential foods made possible further major increases in brain size.24
Continuing his remarks quoted above, John Maynard Smith notes that, “Given sufficient capacity for learning and cultural communication, a population can adapt to its environment by non-genetic means. The mechanisms of history and evolution are so different that it is best to distinguish clearly between them. However, they may interact.”25 With little blurring of concepts, we may substitute the language of “history and evolution” here for “second and first nature.” When it comes to these ideas of scientific critics of the modern synthesis, Bookchin has faithfully translated them (or converged with them) into his own philosophical lexicon. He remains, however, considerably more out of step with the most advanced science when it comes to the modern study of animal behavior.
Social learning: Observations, mechanisms, and concepts
There are a range of definitions of “social learning” in the scientific literature—though as we will see, it is rather less controversial to define than “culture.” Cecilia Heyes influentially defined social learning as “learning that is influenced by observation or interaction with another individual or its products.”26 While subject to certain tradeoffs in evolutionary fitness, social learning is of immense adaptive importance for animals.27 Ecologist Carl Safina writes,
With social learning, an individual who is new and naïve in the corridors of the world gets the keys to the doors and drawers and cabinets of collective knowledge. You get skills tailored to what you happen to need, where you happen to be, as an inheritance from the whole community. It’s a great leap over learning solo by trial and error, that fraught process of acquiring skills at the cost of time, chance, and, sometimes, mortal risk. Social learning is huge, because it means that a dolphin or an elephant, a parrot or a chimpanzee or a lion, can tap into collective skills and wisdom that accrued slowly over centuries. For a young whale: Where in miles and miles and miles of ocean should I look for food? For a young elephant: Where is drinking water when everything I know has dried up? For a young chimpanzee: Now that the fruit is gone, what do I eat? For a young elk: As everything begins freezing solid, where should I go? For a young wolf: How might we hunt and eat this creature that weighs ten times what I weigh? These are all learned skills. For many creatures, they are skills learned from experienced elders.28
Accounts of animal social learning are as old as biology itself. Aristotle compiled the first known evidence of birdsong being learned socially.29 Animal social learning was also widely accepted by the first generation of evolutionists. In The Descent of Man, Charles Darwin attributed it not only to apes (“apes are much given to imitation…and the simple fact previously referred to, that after a time no animal can be caught in the same place by the same sort of trap, shews [sic] that animals learn by experience, and imitate each other’s caution”) but also to dogs, bees, hawks, and other birds.30 Other early evolutionists like Alfred Wallace and George Romanes also put learned traditions front and center as sources of adaptive behavior.31
However, it was some decades before biologists began systematically documenting social learning in animals. The very first scientific studies of social transmission of behaviors were conducted on birds. In 1949, British ornithologists tracked the spread of a practice by blue and gray tits of prying the lids off of milk bottles.32 Others conducted pioneering studies of social learning in birdsong and the resulting variation of dialects by European songbirds in the 1950s and early 1960s.33 These were able to pinpoint transmission to a particular early stage of a young bird’s cognitive development (similar to how humans do most of their language acquisition in a small window of early childhood).34
The most significant early breakthrough came through the studies of Japanese macaques (also known as snow monkeys) led by Kinji Imanishi, now widely recognized as the father of modern primatology.35 Crucially for ethological methodology, he was the first modern zoologist to focus on animals as individuals, tracking the webs of their individual social relationships. In the 1950s, as a result of this established mapping of macaque social networks, his students were able to witness the emergence and social transmission of a new cultural trait in real time. They saw that a young female Japanese macaque named Imo had started using a stream to rinse sand off of her sweet potatoes before eating them. She then progressed to rinsing them in the sea, which not only cleaned sand off but helped flavor the sweet potato with a bit of salt. This behavior spread through pathways of Imo’s personal social relationships. First to pick up the new method was Imo’s child, then her siblings, and then her own mother, and it spread through direct monkey-to-monkey relationships over the course of a little more than a decade until the entire colony, except a few older individuals, were doing it. The technique was first documented in 1953, and today the entire population of Japanese macaques on Koshima Island washes sweet potatoes. An innovation was socially transmitted and then persisted across generations until it became a normative universal characteristic of the population. Building on Imo’s inventiveness, this same group of macaques also learned to do the same thing with wheat, which they lug out to the shore to rinse in the ocean.36
Over the decades since, and especially from the 1990s onward, research on animal cultures has blossomed, opening new frontiers for investigation and theorizing. In Andrew Whiten’s overview of contemporary research on animal culture in Science, he points to the deeper questions we are now able to pose from our current understanding:
Do animal cultures evolve, cumulatively, as human cultures have done so impressively over past millennia? How profoundly does the lifetime reach of culture in animals’ lives reshape our understanding of behavioral ecology and the fundamentals of evolution at large? How close are human and animal cultures now perceived to be, and where do the principal differences remain?37
Today, scientists identify a variety of mechanisms by which social learning can take place.38 Some of these are psychologically quite basic. One animal’s behavior will draw the attention of another to a particular location or object, with which they will then interact through more conventionally individual processes of learning. This is termed local enhancement and stimulus enhancement respectively.39 More complex are learning mechanisms termed “emulation” and “imitation.” Their meanings in the ethological literature are subtly but importantly distinct. Emulation is goal-oriented copying of another’s behavior: the learner sees what another is doing and to what end and tries to figure out how to do it themselves. Imitation is process-oriented copying, precisely following each step. Despite our use of the verb “ape” to refer to imitative behavior, research suggests that humans are far more prone to imitation (as opposed to emulation) than chimpanzees are.40 Many researchers of social learning argue that imitation is a mechanism much more capable of producing enduring cultural practices and is necessary for the development of cumulative culture—which we will discuss shortly.41
The most cognitively advanced social learning mechanism is teaching. It is defined as behavior of a knowledgeable individual who modifies their behavior in the presence of a naïve individual and incurs some cost or derives no immediate benefit from this modification, with the result that the naïve individual learns from this modified behavior.42 Unlike other mechanisms where only the learner is actively engaged in the process of social transmission, teaching is a two-way street. While much rarer than other mechanisms, teaching has been observed among a range of species, including two of our case studies.43 These teaching behaviors seem to rely on remarkably sophisticated psychological aptitudes: a developmental theory of mind, conscious cooperation, and long-term forethought or mental time travel on the part of the teaching adult.
Social learning, as defined by these and other processes, is not precisely (or at least not necessarily) the same thing as culture. Definitions of culture abound and remain hotly contested, within and across academic disciplines. Anthropological definitions broadly presume its limitation to human beings—foundational to the field is Edward B. Taylor’s definition as “that complex whole which includes knowledge, belief, art, law, morals, custom, and any other capabilities and habits acquired by man as a member of society.”44 From the biological sciences, I would point to several complementary ones. Richerson and Boyd define it as “information capable of affecting individuals’ behavior that they acquire from other members of their species through teaching, imitation, and other forms of social transmission.” Similarly, cetacean biologists Luke Rendell and Hal Whitehead describe it as “Information or behavior—shared within a community—which is acquired from conspecifics through some form of social learning.”
Alternatively, primatologist Andrew Whiten suggests a sort of stepped pyramid of concepts with social learning at its base. Social learning, as discussed, refers to any behavior or information picked up by an animal from others. That social learning becomes a tradition when it is faithfully transmitted across multiple generations. For example, when researchers showed female fruit flies a mating encounter between a male marked with a green dye and another female, those onlooking females learned a mating preference for green-dotted males.46 This preference continued to transmit through the population as a tradition and remained in place several generations later.
Whiten argues, as do some others, that we should narrow usage of the word “culture” for reference to a given clustering of multiple traditions that are passed on. Chimpanzees in Kibale National Park, for example, engage in handclasp grooming, the rain dance, and use of stick tools with modified tips to pull water and honey from cavities in trees. The chimpanzees of Bossou, however, do none of these things. Their particular repertoire of traditions includes using stone hammers, fishing for ants and termites, and attracting the attention of potential sexual partners by stomping plant stems underfoot (none of which are seen among Kibale chimpanzees).47 Whiten would classify these each as “a culture,” given that there is a whole suite of socially transmitted behaviors that continue to be passed on. Others assert that the meanings of “culture” and “tradition” are largely interchangeable.48 Many scientists define culture in a manner that includes all mechanisms of social learning, while others assert that applying the word “culture” meaningfully only entails behaviors learned through imitation, emulation, and teaching.49 Summarizing their basic commonalities across disagreements, however, primatologist William McGrew writes that, “Consensually, all seem to agree that culture is learned (rather than instinctive), social (rather than solitary), normative (rather than plastic), and collective (rather than idiosyncratic).”50
At the peak of Whiten’s pyramid of social learning is what we call “cumulative culture.”51 Humans are the clearest case of cumulative culture. What we learn from each other are not singular behavioral innovations but innovative modifications to previous innovations. The result is an accumulation of cultural practices and knowledge that would be simply impossible for any given individual to innovate on their own.52 This cumulative culture has a history and a developmental trajectory. In the famous words of Isaac Newton, “If I have seen further than others, it is by standing on the shoulders of giants.” All human technological advancement relies on this principle, which Michael Tomasello theorized as the “ratchet effect.”53 In particular, cumulative culture seems to rely upon imitation and teaching as key transmission mechanisms. It is not, Peter Richerson and Robert Boyd write, simply “a by-product of intelligence and social life.”54
Cumulative culture among nonhuman animals is a controversial proposition.55 Birdsong is widely recognized as a product of cumulative culture, but few others receive this begrudging consensus. It is certainly much, much rarer than culture more broadly. I do however believe that there are multiple examples of complex animal behaviors we have observed that are simply inexplicable unless attributed to a process of cultural accumulation. I will briefly note two examples of this in the humpback whale: their songs and some of their foraging techniques.
To attract mates, humpback males sing beautiful, complex songs structured by a specific stereotyped pattern of nested phrases and themes, sometimes as much as thirty minutes in length (after which the song is repeated). These songs come into and fall out of style like top forty hits on the radio: for a time, about a year or two, everyone is singing the same song, but it is then replaced by the newest fad, typically introduced by a neighboring humpback population.56 The new song is either a modification upon the song they currently sing, called a “song evolution,” or wholesale replacement, called a “song revolution.” The songs are so internally complex that it is highly unlikely they are composed from scratch by a single singer, and there are recordings of humpbacks singing hybrid songs in the middle of their way to learning or composing a new one, a transition through which segments of the previous year’s are replaced.57 They are not single innovations that spread through a population.
Crews of humpbacks in the north Pacific also engage in a sophisticated cooperative foraging strategy known as “bubble net feeding,” specialized for hunting shoals of small fish at the surface.58 One or more whales give their hunting cry from below, chasing the school of fish to the surface. Others release a stream of bubbles from their blowhole in a conical spiral to surround the fish.59 On the perimeter of this “bubble net” are the “herder” whales in the crew, who bat their pectoral flippers like wings to frighten the fish into remaining within the closing trap. Each whale then takes its turn lunging up through the tight ball of fish with an open mouth, breaking the surface and swallowing huge numbers of them at once.60
Bubble net crews can vary in size, from three or four to as many as thirty individuals.61 “Bubblers,” “trumpeters,” and “herders,” as they are called, tend to maintain their role and their position relative to other members of their consistent crew. Because of the necessity of social learning and close, durable social bonds for this kind of behavior, it was hypothesized that the members of a given bubble net crew would be related, but subsequent genetic testing and tail fluke photo identification confirmed that this was not the case. These associations are based on something other than kinship, and nor are they random. The same individuals come back together for this teamwork season after season, decade after decade. Trying to make sense of the long-term stability of these bubble net crews, one humpback researcher said, “It is intensely human-like… enduring social bonds between non-relatives, engaging in task specialization, and a form of collaborative tool use. We really don’t have any better examples, other than humans.”62
Though speculative, the claim that bubble net feeding is an instance of cumulative culture is a compelling one. It has all the hallmarks of a foraging strategy developed through successive modification. Each of the roles in the crew enhance foraging success, but we could imagine just one of them—for instance, a whale individually disorienting and clumping together a school of fish with a stream of bubbles—being a workable foundation, upon which the cooperative element with the other roles were later elaborations. We can reasonably infer that this is the case based on observations of humpbacks in the north Atlantic, who do exactly this: using spirals of bubbles individually to enhance their foraging strategy, without the trumpeting or herding aspect.63 Most excitingly, humpback researchers on Cape Cod documented a socially transmitted elaboration upon the individual bubble net feeding strategy in real time.
One whale was witnessed in 1980 slamming their tail down on the surface of the water before creating its stream of bubbles to surround the fish. Over the course of the 1980s, this behavior (known as “lobtail feeding”) spread through the population, especially among younger whales. Social mapping and mathematical modeling of the spread demonstrated that it was proceeding through networked social transmission, horizontally and obliquely, and was best accounted for with wave-of-advance models from social science.64 At minimum, we know that lobtail feeding is an example of cumulative culture, which strongly supports the hypothesis that cooperative bubble net feeding is as well—just one that took the baseline practice of bubble-assisted hunting in a different direction.
Part of the difficulty is that cumulative culture is, definitionally, a historical subject of study. It requires long-term observation to identify and describe, yet we have only been searching for it in other species in such a brief window of natural history. Scientific observations to document new behaviors like those at Cape Cod can help us understand how a complex behavior like bubble net feeding might have culturally developed historically, by incremental modifications on one’s learned culture. Through interacting with and learning from one another, humpbacks are developing foraging strategies that are progressively more complex, with innovations piled upon what has been learned from previous generations.
Precise cleavages between this social evolution and the biological evolution it stems from can’t be easily pointed to, but when certain key pieces of the puzzle are put in place biologically (sociality, social learning, and psychological mechanisms for innovation and cultural transmission), they set in motion a novel process of historical development and change that can no longer be fully or adequately explained by the mechanisms of natural selection. This, I argue, is how we would characterize a scientifically robust conception of second nature.
Social learning research methods
Alongside the debates on what social learning and culture are, debates about how we identify social learning in animals are even more contentious. Laboratory experiments to demonstrate it are relatively straightforward. Many experiments utilize what is called an “observer-demonstrator paradigm,” where the aptitude at a given novel task of a control group is compared to that of a group allowed to observe a member of their species demonstrate it first. Other studies use a “transmission chain” framework: two individuals are each taught a different solution to a given problem and then released among their untrained fellows. The study traces the method used by the downstream groups of learners to see whether each group would continue to use their specific method even over multiple degrees of separation. These have shown stable social transmission of a learned behavior in at least twenty different species, and not simply among primates and other large-brained mammals. Rodents, birds, and fish have all been the successful subjects of transmission chain studies.65
Much more complicated to confirm is whether any given behavior seen among free-living populations is socially learned. Simply because a given species has the known capacity to learn socially does not mean all of its behaviors are so acquired. For no species would this be true, not even humans—in essentially all animal species there are also behaviors that are individually learned over the course of interacting with one’s surroundings or that are instinctive or otherwise biologically determined. Many behaviors are the products of intermingling between these and social learning. Humans have evolved the behavior of spoken language, but whether the language a given person speaks is Swedish or Swahili is entirely shaped by social learning. How then are we practically to assess which animal behaviors are acquired via social learning and which have other sources?
Most attempts to do this rely on some version of what is called the “method of elimination.”66 In essence, this approach boils down to demonstrating behavioral variations between two groups of a given species are not explainable by either genetic or local ecological variation—leaving cultural variation as the only remaining possibility. The multi-site study led by Andrew Whiten and Jane Goodall comparing documented behaviors of different chimpanzee populations that concluded widespread cultural variation was present relied on this method.67
This approach has significant problems, however. We cannot actually observe the precise learning mechanism behind a given behavior. Some animal culture skeptics suggest that improper use of the elimination method could lead to false positives (identifying a given behavior as cultural when it has some other source). Few field researchers find this worry persuasive, however. Far more likely with the elimination method are false negatives: failing to identify a genuinely socially learned behavior as cultural because its distribution also maps onto genetic or environmental variation.68 This does not make the elimination method useless, but it is, in the words of Whitehead and Rendell, “a tricky beast, which for intellectual health and safety must always be labeled ‘Handle with care!’”69 Various other statistical methods for identifying social learning in wild animal populations have been proposed and explored, which may approach these problems with more precision with the right observational data.70
Many of these scientific debates about the scope and reach of animal culture have revolved around the chimpanzee. This makes a certain sense: if we want to unpack what might make humans different from other species, we should line ourselves up alongside our closest extant cousin. On one side ethologists in the field point to their suites of highly variable chimpanzee behaviors, both foraging-related and arbitrary social ones. On the other side, experimental psychologists note their relatively undeveloped capacities for imitative learning, being more competent as individual problem solvers. Other research has found that chimpanzees are most likely to adopt behaviors they see in socially dominant individuals, but that most behavioral innovations are created by those low on the social ladder.71 The social despotism of most chimpanzee communities may place significant limits on their cultural development. We have also uncovered chimpanzee archaeological sites, with nut-cracking stone hammers and anvils dating back 4,300 years. This is of course extraordinary in its own right, as well as an indicator that chimpanzee technology has remained essentially unchanged (at least their stone tools) in that region for, at minimum, thousands of years.72
This debate about chimpanzees often circles back to the question of homology versus analogy: in essence, do the cultural aspects of humans and the cultural aspects of other animals actually emerge from the same evolved cognitive apparatus? Or are they only evolutionarily converging and appearing to be similar without direct relationship by descent?73 I consider this debate to be a thoroughly unilluminating distraction, and a narcissistic one at that: reducing scientific import to whether it can tell us something about “where we came from.”
To sidestep the messiness of the “chimpanzee culture wars” and the homology-analogy debate, I will set aside primates altogether. The three species I have selected for more focused case studies are all ones with whom our last common ancestor roamed the earth before the extinction of the dinosaurs—an ancestor we can be reasonably confident had vastly fewer cultural capacities. Any similarities between the societies of humans, elephants, killer whales, and sperm whales are the results of convergent evolution, being indisputably analogous rather than homologous. That convergence is precisely what is so fascinating about them, and where dialectical naturalism has the most light to shed on fundamental questions. We will return to those after reviewing each of these remarkable animals in turn.
Society on the savanna
In Re-enchanting Humanity, Bookchin discusses the familial-biological roots of human society, which structure and facilitate the emergence of second from first nature. He states that,
The protracted dependence of the human child leads to bonds of life-long commitment, even as the mother becomes occupied with the care of new and younger siblings. Sharing food, collective care-taking for the young, an abiding sense of responsibility to the infirm and to the family group as a whole—all yield a clearly discernible human family structure, to an extent that is largely unknown in chimpanzees, our closest primate relatives…74
I find this passage to be of particular interest, for if you remove the word “human” and reference to primate relatives, these exact words could be found in contemporary scientific journals of ecology and ethology, describing the family life of a number of animals—but none more so than African elephants.75
The social organization of collective care is the single most important feature of elephant life. They have other remarkable cognitive capacities—massive and complex brains, extraordinary geospatial and social memories, proficiency in crafting and using tools, an apparent grasp of the concept of death—but Bookchin is onto something very important in seeing the communal family structure as the key biological foundation for second nature.76 Acculturated in family life, elephants create and navigate intricate social worlds. Elephants are slow-growing, slow-developing animals with multiple years between their pregnancies, requiring a high investment in individual offspring to get them through their long period of childhood dependency. They nurse for two to four years, with about ten to sixteen years of social dependence on their mothers. Elephants rely on collective care of their calves, where adults play essential caretaker roles to young who are not their own offspring, a phenomenon biologists call “allomothering.”77 This kind of social organization has various other adaptive outcomes, where individuals are able to rely on one another collectively for survival, and where vast stores of accumulated ancestral ecological knowledge can be maintained in the minds of elders, expanded through group experience, and transmitted across generations.
Elephants exist in fission-fusion societies.78 These are systems characterized by nested scales of social organization that come together and split apart—a means of adaptively balancing the benefits of large social groups (cooperation for predator defense, knowledge sharing, child rearing, etc.) against the problems of increased competition for resources. The family—related adult females and their children traveling and mothering together—is the most basic unit of this fission-fusion society.79 Each family is led by a matriarch, who is typically (but not necessarily always) the eldest female.80 Leadership in an elephant family is primarily about decision-making about where they will travel, but is also of importance for social mediation, cultivating relationships with other families, and serving as a respected source of guidance for younger family members in all areas of life.
At a scale larger than the family is the “bond group.” This is the term elephant researchers use for the close associations that exist between two or three different elephant families.81 Bond groups often travel together and spend significant time in each other’s company. Elephants in different families of a given bond group will care for one another’s babies and even occasionally nurse them.82 When reunited after a period of separation, families in the same bond group express jubilance and excitement. These bond groups are not “next in kin” beyond the family unit, as we might assume. They typically are not related at all, being more akin to “family friends” than second cousins.83 Bond groups seem to persist intergenerationally. Different families’ matriarchs with tight bonds with each other facilitate the social context in which their children and grandchildren develop their own bonds with those of the other family.
Beyond the bond group exists a unit of social belonging that researchers term a “clan.”84 Clans share a home range and assemble together during the wet season, when food and water are more plentiful, before dispersing outwards across immense distances during the dry season.85 They are made up of many hundreds of individual elephants, and this social complexity seems to be a key mover of elephant cognitive powers. Richard Byrne and Lucy Bates (two leading elephant scientists) write that “It is therefore possible that each individual elephant knows and differentiates among several hundred other individual elephants, far in excess of anything found in non-human primates.”86
Navigating the complex and sprawling social world of elephants requires well-developed capabilities of communication and individual recognition. Experimental playbacks of known elephant voices in Amboseli National Park confirmed that average adult female elephants were familiar with and able to discriminate the vocalizations of at least one hundred other individual elephants, the vast majority of whom belong to other elephant families.87 Their responses mapped onto their association indices with the elephants whose vocalizations they heard: calling in response and approaching the loudspeaker when they have a prior familiar relationship, clumping more tightly together and retreating when unfamiliar.
Elephant vocalizations can be produced with their larynx (termed “rumbles”) or with their trunk (termed “trumpets”). Based on extensive field observations, elephant researchers are actually able to “translate” many of the common vocalizations, with contextually consistent usages enabling their classification as little-greeting-rumbles, the let’s-go call, etc.88 Some overlapping vocalizations between individuals appear to be genuine conversations for the purposes of group decision-making.89 There is further strong evidence that these elephant vocalizations are socially learned. One key indicator of this is vocal imitation, which elephants are quite capable of. Some African elephants, for instance, have learned to mimic the sounds of trucks.90 Harvey Croze and Cynthia Moss write that,
The fission-fusion society of elephants is built upon a complex network of social relationships within and between families, bond groups, and clans and between individual males. Added to this multi-layered social network are fleeting interactions and temporary consortships that form between reproductively active males and cycling females. This elaborate system of associations, partnerships, coalitions, and enduring relationships is in part established, mediated, and maintained via an intricate suite of acoustic signals.91
Beyond these two modes of vocal communication that we can hear, however, elephants also rely on others outside of our sensory abilities to detect.
Only in the late ‘90s was it discovered that elephants utilize seismic communications, producing low frequency infrasound (i.e. below what human ears can hear) that travels through the ground rather than the air. These seismic rumbles can be “heard” many miles away from their source, where the receiving elephant absorbs them through their feet.92 Somewhat speculatively, it is thought that this might explain the many past accounts of inexplicable elephant behavior that seemed to rely on them receiving warnings from afar.93 As of yet, we have little grasp of what sorts of information they are able to transmit with infrasound.
Lastly, elephants possess an extensive repertoire of chemical communications. They have unbelievable powers of smell, the most sensitive and precise of any animal, and in addition to their ability to recognize specific individuals by their voices, they can also recognize individuals by their unique chemical signals in urine, temporal glands, sweat, exhalations, and other bodily sources.94 We tend to think of smell more as a sensory source about what is “out there,” a one-way mode of information gathering, but elephants also “speak” through scent, generating chemical signals that convey information to others. Secretions from their temporal gland, a “large multi-lobed sac with an orifice mid-way between the ear and eye,” are key for this.95 Temporal lobe secretions are visibly observable as well—elephant researchers report the sides of their heads streaming with fluid during moments of emotional arousal.96 Although we can barely begin to understand this aspect of their communication, we know that at minimum these communicate extraordinary amounts of important information: emotional states like aggression, calm, distress, or relief; states of sexual arousal; and perhaps individual and group identity.
Alongside the necessities of communal child-rearing, another biological underpinning of elephant social forms is their extreme degree of sexual dimorphism.97 Male elephants inhabit an entirely different social world. When they reach adolescence in their teenage years, males leave their natal family behind (and are sometimes pushed away). They travel in small groups of other young males, encountering and learning how to be in the world from older males as they await their age of active reproduction. They become sexually mature around age twenty, but few males ever reproduce before the age of thirty. Older males reach truly enormous sizes over the course of decades, two or even three times as massive as full-grown females, and the largest males are the most desired mates. Fully mature males periodically enter a state called “musth,” where their entire body is flooded with extreme levels of testosterone, sixty times higher or more than their normal state, making them highly aggressive and highly motivated to find mates.98
These huge male elders are needed to circulate genetic material across enormous distances, but they also play an important role shaping the social development of young roving males, who they regulate hormonally and for whom they are essential role models. Their social importance was not apparent to us until faced with the consequences of their absence. Lacking their guidance, younger males become disorderly, confused, and violent. In the 1990s, a handful of orphaned young adult males were sent off to a park in South Africa that had no other male elephants. They went on a killing spree, goring more than one hundred rhinos to death for no reason. Without the hormonal suppression and social leadership that older males would have provided them, these young males entered musth prematurely and had no understanding of how to direct their explosive aggression. A South African ecologist proposed releasing fully mature males into the park with them, which successfully halted the rhino killings.99
Despite sex being among the most presumably instinctive of animal behaviors, young elephants also require sex education: specifically how to act to invite sexual encounters and who to seek out as a mate. Female elephants reach sexual maturity around ten to twelve years of age. When not pregnant or nursing, they periodically go into estrus, a heightened hormonal state lasting several months punctuated by ovulation. Estrus females perform a sort of seductive ambling walk with tail held high called the “estrus walk,” which we understand as a flirtatious invitation to males. This is not an instinctive behavior, however. A young female entering estrus for the first time does not know how to do it and needs to be taught: older females in her family start to act out the walk for her even though they themselves are not in estrus—to show her how it is done.100 Guidance from one’s mother, grandmother, aunts, or older cousins is also important for learning who is best to mate with. The largest, oldest elephant males who have entered musth are seen by mature females as the most attractive mates, but this preference must actually be taught to a young female.101 They appear the most physically intimidating, hyper-loaded with testosterone and perhaps three times her size, and left to her own devices she might opt for a partner closer to her in stature and more muted in his aggression. This, however, would be a potentially dangerous mistake. Smaller, less experienced males sometimes injure (occasionally permanently) the females they mount by displacing their weight onto them, while the massive older males are strong enough to support their own weight on their hind legs. A more physically dominant partner will also scare off other suitors, preventing any possibly hazardous altercations from males vying for mating access.
Similar sex education takes place in the social world of males. Young males tag along with their elders in musth, copying them as they smell females’ urine and maneuver around them. Figuring out who is actually in estrus (rather than pre-estrus, or even having recently given birth) based on urine scent is a learning process in which uninitiated youngsters often make mistakes. Counterintuitively, older males in musth are perfectly welcoming of these learners, perhaps because they are so unthreatening at that stage. When a musth male is guarding an estrus female, he will keep all other suitors at a distance, allowing none to approach within tens of meters, but he will tolerate one of his young neophytes to stand right alongside her.102
This guided entry into the world of reproduction only deepens when it comes to raising the resulting calves. A juvenile female learns how to raise future young of her own by assisting older females in her family with their calves, so that babysitting also functions as a mothering apprenticeship. When she gives birth to her first calf, those more experienced females will come to her aid in turn. Observationally, it appears that mature female elephants understand that their daughters, nieces, and cousins who are first time mothers have less experience, because they provide them with much more calf-rearing support than they do for the older mothers in their family.103
These relationships of care are foundational to elephant social life, particularly female social life. We observe them helping to prop up the exhausted and lift the fallen, pulling spears or darts from the flesh of their comrades, slowing the pace of the group’s travel to match what an injured member is capable of, and even placing food directly into the mouth of another elephant with an injured trunk.104 This, along with their extended childhoods, is the relational fabric through which elephant acculturation takes place, mirroring the rich human sociality that Bookchin mistakenly declares is the province of our species alone.
Conflict resolution to maintain these relationships of care is typically mediated by a family’s matriarch or other trusted third party. When some sort of aggressive altercation occurs, like rough pushing, followed by a protesting call by the offended party, the matriarch will approach, using specific reconciliation vocalizations and body language and reach out to each with her trunk. The matriarch may call over other family members (like the mothers of the conflicting parties), who continue with reassuring rumbles. After a bit of grumbling, the matter is quietly resolved.105
In addition to the cultural processes required to reproduce their social worlds, culture is also vital to elephant survival in a complex and climatically variable ecology. Savannah elephants migrate extensively, relying on the decades of knowledge and historical memory of their elders to find water across ancestral migratory routes. This knowledge, passed down through elephant families, is a matter of life and death, especially in our new era of devastating climatic changes.
In 2008 and 2009, a horrific drought killed hundreds of elephants in east Africa. This climate disaster, however, did not strike elephant families equally. Those with younger matriarchs were devastated. One family lost twenty members. The KA family in Amboseli, led by a pair of matriarchs ages thirty-nine and forty respectively, however, made it through the drought without a single death.106 What made the difference was whether a family had a matriarch who remembered the last drought of that magnitude decades earlier, when their own mother or grandmother would have led them to one of the last remaining sources of water—sometimes requiring hundreds of miles of travel to reach.107 Elephants have been observed to change course after a period of unexpected rainfall to seek out remote water sources along routes that they had not used for years.108 The extraordinary landscape knowledge of older females is a product of the interaction between their evolved powers of memory and social learning. These work together to socially accumulate information about drought-resistant sources of food and water over deep time, passed across generations of grandmothers, mothers, and daughters.
For elephants, their physical strength and endurance, their extraordinary senses, and even their wits, all transmitted to them by natural selection, are inadequate for survival. Their social and ecological world rests upon unbroken chains of cultural continuity with their ancestors, which each successive generation of grandmothers tap into and add to with their own experiences.
Cultures beneath the waves
The world of dolphins is one shaped by an incredible array of social learning and cultural practices, of which we have an extensive and ever-growing body of observations. They exhibit highly variable complex behaviors and build rich social lives with one another. Dolphins learn from one another an amazing diversity of foraging strategies, many of which are particular to a given community.109 Cooperative fishing practices between dolphins and humans have developed independently at least three times, in Brazil, Mauritania, and Australia, using distinct methods.110 Extended periods of childhood accompaniment of calves and mothers and socially complex fission-fusion societies present many opportunities for social learning in all dolphin species. Their vocalizations, ways of socializing, divisions of labor, and group identities all also appear to be cultural.111 One dolphin species in particular, however, demonstrates their highly developed cultural systems in some of the most vivid ways in the animal kingdom: the killer whale.112 Killer whales are one of the most unambiguous cases of animal biology being non-determinative, in which members of the same species have societies with radically different structures and lifeways on the basis of family and geography.
Killer whales are intensely social beings. Feeding, mating, and caring for and teaching their young are all collective experiences. Even giving birth, in some groups, involves multiple females helping to bring the new baby to the surface for its first breath. Some newborns bear teeth markings that suggest another female helped pull them from the birth canal like a midwife.113 These relationships of care have rooted themselves in the female life cycle. They are among only five animal species known to have evolved menopause, the others being beluga whales, narwhals, short-finned pilot whales, and of course humans.114 Despite ceasing reproduction sometime in their forties, female killer whales typically live for seventy or eighty years, and in some cases for more than one hundred years. Menopause evolves when the reproductive advantage of continuing to attempt to produce more young (balanced against the additional competition for resources introduced into the family group) is eclipsed by the contribution grandmothers can make to the survival of their grandchildren and great-grandchildren as secondary caregivers and food providers and as custodians of ecological-cultural knowledge. For killer whales, the importance of this in the enhanced survival of calves is known as the “grandmother effect.”115 In some ways mirroring Bookchin’s ideas, Jared Diamond and others have argued that menopause is as important to the evolution of human societies as large brains and bipedalism.116 The same seems to be true of killer whales.117 While their social structures are incredibly varied, grandmothers are always at the center.
They are also the top ocean predator, capable of preying upon almost anything, up to and including great white sharks, other dolphin species, and whales, with no natural predators of their own. They live in every ocean, from Greenland to Hawaii to Indonesia to Antarctica. Their communities have culturally transmitted prey specializations, with some teaching their young the arts of hunting seals or herring or penguins. We know a remarkable amount about some of these killer whale populations, based on decades of continuous observation, using individual identification to map their social lives. There are others about which we, frustratingly, know almost nothing at all. I will lay out a light overview of what we do know, which—while painfully limited—is more than enough to indicate the incredible cultural diversity of this global species.
The most well-studied population of killer whales in the world are those living off of the coast of the northwest United States and British Columbia: specifically the populations known as “residents.” Resident killer whales are an example of what are called killer whale “ecotypes,” a categorization of their particular ecological niche that differentiates them from the other ecotypes. The resident ecotype exclusively preys upon salmon, and overwhelmingly eats a single species of salmon (the Chinook, or king, salmon, which alone makes up about 80% of their diet). Food sharing is a universal behavior among residents, both from mothers to their immature offspring and between adults. For animals nearly the size of a bus, holding a single salmon in their teeth for another to feed from—a both extraordinary and touching image—is likely of more social than caloric importance and plays a role in the social learning of their strong prey preferences. As their name suggests, resident killer whales remain in these waters year round, moving between different salmon runs based on their historic seasonal peaks in abundance—drawing upon the accumulated ecological knowledge of their matriarchs.
Like African elephants, resident killer whales have a social order built around nested groupings of kinship and affiliation, though we can more clearly piece together the social markers and identities structuring it than is so far possible with elephants. The most basic social unit is the matriline: an elder female killer whale (a matriarch), her children, her grandchildren, and occasionally great-grandchildren. These children stay with their mothers for their entire lives (including the males, quite unlike elephants). Multiple related matrilines travel together in what is known as a “pod,” where most resident killer whales spend most of their time.118
Resident pods share a common “vocal dialect,” with distinctive patterns of vocalizations that are socially learned.119 Killer whale vocalizations fall into three broad categories that are produced in different ways: “discrete calls,” which have a harmonic structure that is most distinctly “orca” to the human ear and which they use in socializing with one another; “clicks,” which are the sounds they use for echolocation; and “whistles,” which are also used for social communication.120 Resident killer whale matrilines will also often have their own distinctive call.
The next level of social organization is the clan, made up of pods who share certain vocalization types and who frequently associate with each other.121 Pod vocal dialects within a clan are roughly equivalent to distinct accents, where everyone can tell where a given individual is from by certain identity markers in their voice, but their communications remain mutually intelligible.
The residents are an excellent example of killer whale “ecotypes” not being coterminous with killer whale “cultures,” however—though these ecotypes are of course clearly cultural. That is because there is a fourth layer of social organization among the residents referred to as a “community.” There are three communities of resident killer whales along the North American Pacific coast: the southern residents, the northern residents, and the Alaska residents. Their ecotypical behaviors are identical: all three hunt salmon near-exclusively, with most of their diet composed only of Chinook salmon; they all remain in the region year-round, with overlapping ranges; they are all highly vocal (in contrast with other killer whale ecotypes, some of which have radically different vocal behaviors); they are organized into parallel matrilinear social systems of pods and clans with elder-female led families; and they have no discernible physical differences.122 However, their vocal dialects are much more sharply distinct from each other, and they never associate with one another. Each possesses a sharply distinct cultural identity, to a xenophobic degree. Their self-segregation is more stable than any social arrangement in known human history.
The different resident communities also perform some unique behaviors. The northern residents alone make use of “rubbing beaches.” They have several shallow locations of smooth pebbles and sandy patches where they gather together to rub their bodies against the floor.123 Whether this is adaptive in some way or an arbitrary social ritual is currently impossible to say, but other killer whales passing through their rubbing beaches make no use of them. When different pods of southern residents encounter each other, they hold what researchers call a “greeting ceremony,” forming up fin to fin in a straight line at the surface before approaching the other group. When they reach each other, the ritual orderliness comes to an end and they frolic and vocalize excitedly. Fads of wearing a dead salmon on top of their heads spread like wildfire among southern residents in the past, but this has never been seen among the northern or Alaskan residents.124 The southerners also breach the most among residents.125
Within a given community, killer whales aim to mate with those least closely related to them to limit inbreeding, which they assess by how distant their vocal dialects are.126 Mating happens when different pods come together for mass socializing, not within pods. But despite their excitable promiscuity, resident killer whales never mate across community lines, and have remained genetically isolated from one another for some thousands of years.
Across an even greater cultural chasm lie the second ecotype of the northeast Pacific: the transients.127 These migrate seasonally over thousands of miles hunting marine mammals, in much smaller social units than residents. Transients and residents never interact: sometimes actively avoiding each other, more often simply ignoring each other.128 Mitochondrial genetic evidence suggests that the maternal lines of residents and transients have been diverged from one another into wholly or partially reproductively isolated populations for at least 35,000 years.129 While still matrilineal, the social structures of the transients bear little resemblance to those of their piscivore cousins. Each pod is composed of only a single matriline, with typically three to seven adult members. Residents remain in their pod for life, while transients occasionally out-migrate from theirs to form a new pod.130 Their vocalization behaviors and the vocalizations themselves are also radically different. Unlike the residents, who chatter to one another endlessly, transients remain deathly silent while on the hunt, using only infrequent echolocation clicks so as to not alert their prey to their presence.131 Big, loud groups help find salmon, while small, quiet ones are better suited to sneaking up on seals. They “apparently refine their social systems” around their ecological way of life.132
Transient populations in the North Pacific rely on historically accumulated knowledge of mammal migrations and life cycles, returning to particular areas in different times of the year when prey is most abundant. In the spring, gray whale mothers migrate north from the Gulf of California with their calves to the rich feeding grounds of Alaska. Transient killer whales have learned to hunt gray whale calves by separating them from their mothers and holding them underwater to drown them. In May and June, northern fur seals arrive to the Pribilof Islands to give birth and mate, where the transients will remain until early July.133 The AT1 group (awaiting their extinction as a result of the 1989 Exxon-Valdez oil spill, since which no babies have been born into AT1) and the GOA (Gulf of Alaska) group are two distinct and culturally isolated populations of transients hunting the same prey through the same waters, but do not interbreed or associate with one another.134 There are also the West Coast transients, who travel between the coasts of California and British Columbia. These primarily hunt harbor seals.135 Transients also spend at least some time of the year in the deep subtropical region of the North Pacific, judging by limited satellite tag data and the unique scars some of them bear from the bites of cookiecutter sharks, which live only in the tropics and subtropics.136
Third and least well-understood in the North Pacific are the offshore killer whales. They hunt a variety of large fish species out in the deep ocean, with such a strong preference for sharks that the points of their teeth are worn down by puncturing their sandpaper hides. Offshore killer whales travel even greater distances than the transients—some individuals spotted off the coast of California have also been seen as far north as the Bering Sea.137 Their social structures are difficult to ascertain but seemingly fluid, with traveling companions constantly changing. At times they are seen in small groups of around five individuals; at others they assemble into massive crowds of over one hundred.138 Social network maps of the offshores are dense and chaotic.139 They are highly vocal like the residents, but with much diverged and generally higher-pitched vocalizations. Genomic evidence places them as much closer in ancestry to the residents than the transients. Much more research is needed to unravel the complexities of their social world, though their immense range poses a significant challenge to sustained observation.
We are only beginning to understand the societies of killer whale ecotypes outside of the North Pacific. Those inhabiting the waters around Iceland are much more capable prey generalists than their cousins in the Pacific. Though they most often cooperatively hunt schools of herring by herding them into tight balls and stunning them with their tails, they also have been seen hunting birds, squid, and sea lions.140 Social structure shifts seasonally: during the migration of herring in summer and winter, the Icelandic killer whales gather in huge numbers, while at other times of year they disperse into other prey specializations in different group sizes. Some continue to follow the herring; others swim across the sea to hunt Scottish sea lions. While theirs is also some variety of multiscalar society, it has no clear nested structure of matrilines within pods within clans within communities that we see among the Pacific Northwest residents.141
Other ecotypes elsewhere around the world are observed, proposed, and contested. There may be a second north Atlantic ecotype specializing in hunting dolphins and minke whales.142 In the southern hemisphere, killer whales are much less well-studied, but there do appear to be distinct ecotypical populations among the 25,000 or so in Antarctic waters.143 A “Type A” variety tracks minke whales through ice-free water offshore.145 When a target is identified, the hunter assembles and confers with their podmates some distance away from the ice, and they swim in a line towards the seal. With synchronized tail movements, they create a three-foot wave that washes over the ice and knocks the unsuspecting seal into the ocean where it is pulled under and drowned.146 A smaller-bodied Type B2 generalist hunts in large groups that attack penguins, fish, and the occasional seal. Both Type Bs also make periodic nonseasonal migrations from the Antarctic Peninsula to subtropical waters offshore from Uruguay and Brazil—the current prevailing hypothesis is that this is a strategy for skin repair rather than any mating, birthing, or foraging behavior.147 Type C hunts Antarctic toothfish in the Ross Sea, and Type D (distinguished by its exceptionally small eyepatch and more bulbous head shape) swims in sub-Antarctic waters seeking unknown prey.
In Hawaii, killer whales hunt prey as varied as squid and humpback whales, although we have little reason to believe that these prey types correspond to distinct specialist populations.148 Patchier datasets from the Pacific coasts of Mexico, Patagonia, the Mediterranean, the southern Indian Ocean, Marion Island in the Southern Ocean, New Zealand, South Africa, and the Canadian Arctic indicate an immense ecological diversity and correspondingly sprawling differences in social structure and behavior.149
This array of killer whale ecotypes with different ecologies, reproductive isolation from one another, and certain morphological differences suggests the question of species. Is there, in fact, a single species called the killer whale, or many?
This is a fraught and complex question about which there is no scientific consensus, wrapped up as much in semantic messiness about the meaning of “species” as it is in empirical disagreements about the biology of killer whales.150 Taking the “biological species concept” for granted (defined as populations able to produce fertile young with one another), however, all evidence suggests that killer whales remain a single species. Widely diverged ecotypes have been bred together successfully in captivity.151 What is clear, however, is that there are nonetheless real morphological differences between ecotypes—prime candidates for gene-culture coevolution. And recognizing the reach of social learning in killer whale behavior and thereby the different selective pressures culturally distinct populations face forces us to rethink their possible speciation.
Ernst Mayr, the most influential evolutionary biologist of the twentieth century, maintained that speciation could only occur when a given population was geographically separated, after which the two populations on either side of the divide would face different selective pressures over time and eventually become so different that interbreeding would no longer be possible even if they were reunited.152 This became, for a time, the received wisdom of evolutionary biology. That may be surprising, given its utter implausibility as a theoretical framework for understanding the speciation of large, highly mobile marine animals, between whom geographic barriers are quite literally impossible.153 Something else must act as the root cause of their reproductive isolation.
In Becoming Wild, Safina develops a theory that culture is necessary to explain not just killer whale evolutionary divergence but a great many speciation events, given how many species clearly emerged right alongside their close relatives rather than on the other side of a disappeared land bridge or in separate remnants of a dried up lake, and how much existing animal biodiversity outstrips the barriers of geography. He argues that culturally transmitted foraging specializations within a population create subpopulations capable of cooperation. “Cooperation depends on shared expectations,” he writes. “…But between cultures, expectations differ.”154 A feedback loop of gene-culture coevolutionary diversification results: “Cultural segregation prompts further specialization. Then the specializations lead to genetic evolution and actual physical changes. In that scenario, culture leads, and genes follow. Eventually, evolution might drive specialist populations into separate species.”155
This is exactly what seems to be underway with killer whales. After several tens of thousands of years of cultural reproductive isolation, for instance, the north Pacific transients have developed larger and more powerful jaws than the residents: an evolutionary adaptation downstream of their cultural trait of exclusively hunting large mammals.156 This is as clear a case of gene-culture coevolution as lactose-tolerant pastoralists. Safina writes, “These hidden cultural differences have sent killer whale cultural groups on differing evolutionary trajectories worldwide. We tend to think of culture as almost the opposite of genetic evolution. But in many species, culture-based segregation has fundamental evolutionary implications that have been almost entirely overlooked by scientists.”157 This phenomenon, for what it’s worth, is not nearly as “overlooked” as Safina suggests, and he here summarizes the thinking of a good many evolutionary biologists and cetacean researchers.158
I must add that this theory is an important window into the expansive role of gene-culture coevolution in biology, but it also seems to constrain cultural separations within a species to matters of foraging ecology. Reproductive isolation among killer whales is not limited to ecotypes, as the social chasms between the different communities of residents illustrates. Part of what makes cultural variation so fascinating is its frequent seeming arbitrariness, where explanations rooted in behavioral ecology have no real foothold. Vocalizations are the most apparent to us as differences constructing identity and belonging, but so too may ritualized behaviors like the southern resident greeting ceremony and the northern resident rubbing beaches.159 Some researchers have assembled intriguing early evidence that such cultural markers of identity are driven by schismogenesis, social identity being defined against an other, in much the same way as theorized in David Graeber and David Wengrow’s The Dawn of Everything for human societies.160 Long term studies of northern residents, for instance, suggest that their discrete calls shift over time, but not as blind cultural drift. Rather, the matriline-specific calls compared over time were adjusted such that they could continue to be clearly distinguished from each other.161 This makes sense, after all, if their function is to be able to say “who I am” and “with whom I belong.”
A countervailing force against culture-driven speciation we must keep in mind are the very shifts in ecological conditions that make capacity for wide-ranging adaptation necessary in the first place. Rendell points to the cultural innovation among local killer whales off the southeast coast of Australia that produced a cooperative relationship between them and human whalers. The killer whales would herd a baleen whale into the bay and signal to whaling ships by breaching and slapping their tails. The whalers would come, kill the whale, and then leave it suspended underwater for a day to give their killer whale partners a chance to eat its highly desirable tongue and lips.162 This particular practice was only able to continue for less than a century—eventually the great whales were so diminished that killer whales and whalers alike had to move on.163 A culture’s ability to change rapidly is how it contributes to the survival of the animals who make it. Contrast this with residents who have literally starved to death due to a combined lack of Chinook salmon and an unwillingness to eat anything else. While other salmon species have partially recovered in recent decades, Chinook numbers are still perilously low, which is the primary driver of the southern residents’ continued population decline.164 Some killer whale populations are hyperspecialists, while for others being prey generalists is itself part of their culture. In other words, how culturally adaptive versus conservative a given killer whale population is may itself be culturally determined: a challenging conservation morass to be sure.
Lastly, I will note that for superpredators like these, their cultural trajectories can induce trophic cascades that send shockwaves rippling through ecosystems. Prey innovations among some North Pacific transients, for example, brought devastating declines in sea otter populations.165 (Sea otters are small, and it takes many of them to feed a killer whale.) Sea otters, in turn, play a keystone species role in the preservation of highly productive kelp forests, as the primary predator of sea urchins. With the sea otters gone, sea urchin populations exploded and grazed uncontrollably, causing a collapse of the kelp forest ecosystem and its replacement by “urchin barrens.” Worldwide, killer whales exert profound downward population pressure on a huge number of prey species. Possibly in parallel to the megafauna extinctions that accompanied human dispersal around the globe, the evolution of killer whales about ten million years ago was closely followed by the extinction of more than half of Earth’s cetacean, pinniped, and sirenian species.166 Put in other terms, we humans may not be so alone in the content of our second natures having major ecological ramifications after all.
For animals like killer whales, culture is not merely an appendage to their core biological functions. Instead, Whitehead and Rendell argue, for these beings, “culture is vital”: they literally cannot survive independently of these processes of social learning and acculturation. It is as much who they are as their flippers, blubber, and teeth.
Nations of the deep
Last among our species cases is one whose daily life we know least about, but whose potential nonhuman second nature I find most exciting: that of the sperm whale. Like killer whales, sperm whales are a global species, present in every ocean. The sperm whale is the largest and most evolutionarily diverged toothed whale and in possession of the largest brain that has ever evolved on Earth. They live in a vertical world, where the cycles of their existence are shaped by movement between the surface and the crushing depths to which they dive to hunt, for up to an hour and sometimes more than a mile deep. Their prey mainly comprise vast numbers of deepwater squid, which they pursue with the most powerful natural sonar system in the world. The depth at which they spend much of their lives makes them difficult to observe and study, and only in the past twenty-five or so years have we had any real inkling of their social landscape (seascape?).
The basic sexual-social structure of sperm whales is in many ways identical to that of African elephants, despite their evolutionary separation by more than one hundred million years. They too have extreme sexual dimorphism, with males growing to massive sizes, two to three times as heavy as mature females. The heads of males are also proportionately larger, sometimes nearly half of their entire body length. These physical differences are accompanied by sexual segregation: family life is the tight-knit relationships between mothers, sisters, aunts, grandmothers, and their respective immature offspring, while males leave their families (called “units”) behind upon reaching adolescence and form relationships with other unrelated roaming bachelors. These groups of males are also sites of social learning—they have taught each other to pluck fish off of commercial fishermen’s longlines in the Gulf of Alaska, for instance.167 Like young male elephants, they must wait until they attain massive sizes in their thirties and forties before they are considered desirable mates, at which point they become more solitary. This sexual divide is ecological as well. Females and juveniles remain in the tropics, while the males range into the colder waters of the polar north and south.168
While killer whales are typically cooperative hunters, sperm whales are first and foremost cooperative mothers. Female society is a structure primarily for the purpose of sharing childcare responsibilities among adults who cannot be in two places at once. The basic dilemma they face as a consequence of their ecology is that mothers must leave the surface to hunt, but their babies cannot, and they remain highly vulnerable to predators without constant supervision.169 Their social groups as much as their oxygen-packed muscles enable them to access deepwater food sources.
Bookchin noted that our extended period of childhood dependence sets humans apart from other animals, as a source of social collectivity and of acculturation of new generations. But sperm whale young also pass through an extended period of reliance on nursing and collective care. Most often, a sperm whale juvenile lives solely on its mother’s (or other caretaker’s) milk for the first five years of life, but even longer periods are common. The longest documented nursing among sperm whale calves was thirteen years.170 Due to the amount of nutritional and social investment in each calf, sperm whales have the slowest reproduction rate of any cetacean. Whitehead and Rendell write, “The young cetacean becomes a part of its community’s social network, sometimes a central part of it, and learns through exposure how it works… Such social networks are the ideal social substrate for culture. During this long and intensive period of maternal care, the young cetacean is exposed to information about where members of their community go, what they eat, how they hunt, and how they manage their social relationships.”171
There are culturally different systems of allocare in different communities of sperm whales. In the Caribbean, where sperm whales have been studied extensively, females monitor one another’s children to allow the birth mother to dive and feed. But in the Sargasso Sea and in the Indian Ocean, they also nurse one another’s babies: a new level to the collectivization of care.172 One sperm whale (nicknamed Tereka, of Unit T in the Atlantic) has never, as far as we know, given birth herself, but has consistently nursed the calves of her family unit.173 In another unit, different females in the same matriline will nurse each other’s young, but not those who are less immediately related.174 Another similarity to elephants is that sperm whale family units are often part of enduring associations (referred to simply as “groups”) with other units, spending significant amounts of time together—sometimes another source of babysitting reciprocity.175 Also like elephants, their families need to reach consensus about where to travel. Analysis of partial turning motions of sperm whales in the Galapagos suggest that these movements are how group preferences are slowly tallied and these decisions made, democratically.176
Like most cetaceans, sperm whales live in a world of sound, both because sound travels four times faster through seawater than air and because they have to spend so much time at depths where no sunlight penetrates. They generate immensely powerful vibrations by forcing air through what are called “phonic lips” and lipids of different densities in their enormous heads, bouncing those vibrations off the curved upper part of their skull as a radar dish-like reflector, and sending them out into the water as a pulse of sound that can travel for miles.177 This then bounces back off of objects in the water, which the whale detects through ultrasensitive fatty tissues in the lower jaw, and uses to generate detailed instantaneous images in its brain.
In addition to echolocation, these clicks are used for social communication. When encountering one another, sperm whales produce Morse code-like series of patterns with between three and forty rapid clicks, which researchers call “codas.”178 Different groups of sperm whales use different codas that seem to transmit different kinds of information about who they are. Baby whales learn their family’s codas by the time they are around three years old, although before that, they have something akin to a baby babbling period, where they are making coda-like sounds that are mostly nonsense.179
The critical breakthrough in the study of sperm whales came in the late 1990s when Whitehead and Rendell analyzed sperm whale associative indices (essentially social network maps of who hangs out with who) alongside accumulated coda data. Their eureka came with the realization that patterns of association between different sperm whale units aligned precisely with the most common codas they used. This was the discovery of “vocal clans”: the hidden key to unlocking the world of sperm whale society and history.
Clans, we now understand, are vast social agglomerations of cultural identity marking the bounds of cooperation between strangers, defined by vocal dialect. When female sperm whales with no prior relationship encounter each other, they produce a series of clicks that is their clan’s coda. If they find that they and the stranger belong to the same clan, they will gather together, but if they belong to different clans, they will part ways without further communication. Different clans have different but overlapping geographic scopes, which are immense. Hydrophonic data gathering and total population projections suggest that while clans vary in size, some may be made up of tens of thousands of individual whales. Those many thousands of individuals are mostly unrelated and mostly strangers to each other, but they share a common identity that serves as the foundation of possible cooperation—in no other species but humans does “group identit[y] extend so far beyond kin.”180
In the Pacific, we have identified seven distinct vocal clans, with the most cultural diversity clustered around the Galapagos Islands and the coast of Ecuador. The clan with the smallest range spans one thousand kilometers; the one with the largest ten thousand, essentially traversing the entire ocean basin. Every Pacific clan overlaps geographically with other clans.181 There are at least three clans in the Atlantic, who generally do not overlap with each other.182 Preliminary data suggests two (at least) possible clans in the Indian Ocean.183
Unlike with killer whales, we cannot directly observe differences in foraging strategies between distinct sperm whale cultural groups. However, enterprising researchers have gleaned interesting results from a proxy metric: defecation rates. Sperm whales leave brown clouds at the surface, and the more often they do this, the more hunting success we can infer that they have.184 By compiling huge amounts of poop data from sperm whale units of known clan affiliation, scientists have been able to construct a glimpse into the apparently quite substantial variance of each clan’s hunting success. In normal years, the “Regular” clan in the Galápagos (those who introduce themselves with an even “click-click-click-click” coda) have had nearly twice the foraging success of the other main clan they overlap with, the “Plus-Ones.” (The Plus-One coda is “click-click-click-[pause]-click.”) But during El Niño years, when conditions are generally disastrous for marine life in the islands, their differences in relative success are reversed.185 Only 2% of Plus-One dives observed during El Niño were followed by defecations, but among the Regulars it was essentially zero.[/efn_note] We as yet cannot unravel what their different cultural methods of hunting are, but the Plus-Ones apparently forage in a way that hedges against starvation in the worst times. Another indicator of culturally variable foraging strategies is their movements. Regular clan families remain closer to the shore and travel in meandering, wiggly paths. The Plus-Ones hunt farther out at sea and travel point to point in direct lines. The Regulars frequently dive in unison, while Plus-Ones stagger theirs.186 This difference in how they dive might be linked to their respective reproductive success, since having calves in tow requires them to always leave at least one adult at the surface—Plus-One adult females are considerably more likely to be accompanied by a baby than Regulars are.187
Everywhere sperm whales are found, researchers uncover clan affiliations. Whitehead and Rendell write,
…we sailed to other parts of the South Pacific, recording the codas of the sperm whales. We found the same pattern of overlapping clans and could roughly trace their spread. While we only heard the Plus-One clan off the Galápagos and in the neighboring waters off mainland Ecuador, we recorded the Regular coda repertoires in Chilean waters. The Short clan, so rare off the Galápagos, was heard almost everywhere else, including off northern New Zealand, seven thousand kilometers away, right across the Pacific. Off northern Chile, where we had spent nearly a full year studying the sperm whale groups, we found differences between the clans in movements and feeding success like those we had traced off the Galápagos.188
We can confirm, beyond a shadow of a doubt, that clans are not biological, for the simple reason that the only time clan identity is ignored is for mating.189 As with elephants, elder sperm whale males are wandering bachelors, who travel thousands of miles, sometimes crossing from one ocean into another, circulating diverse genetic material. Clan identity is something that is culturally transmitted—there is no other possible mechanism. “[Y]oung sperm whales,” Whitehead and Rendell write, “learn clan-specific behavior…from their mothers and other members of their unit and clan, and then, especially if they are female, these norms govern much of their way of life.”190 And so clans themselves are complex, translocal social institutions, whose closest analogs in the animal kingdom are human ethnolinguistic or national identities.
This is on remarkable display in the sperm whale genome. Their nuclear DNA, which is received from both parents, is diverse and varied even within family units. But their mitochondrial DNA, received exclusively through the maternal line, shows haplotypes common to clans. Diversity in mitochondrial DNA is low at the population level and mostly nonexistent within family units. One plausible implication of the low mitochondrial genetic diversity of the overall species population is that there was not historically equal reproductive success between all cultural groups—that relatively few socially continuous female lines have given rise to the existing culturally diverse population of sperm whales across all oceans, meaning active processes of cultural diversification must be underway. The most successful sperm whale cultures expand into new territories and undergo some kind of process of new identity formation, at a rate far outpacing the accumulation of mitochondrial genetic changes.191 Another clear implication of this genomic data is that social transmission of codas must take place with extremely high fidelity across generations.192
While similarly firmly rooted in the matrilineal context of socialization and based on principles of xenophobic avoidance, sperm whale clans are not wholly impenetrable cultural barriers in the same way killer whale communities are. On some occasions, females leave their clan and are adopted into a unit belonging to a different one, mixing around mitochondrial DNA.193 We do not as yet understand why or even how this takes place. And most unlike parallel institutions among killer whales, sperm whale clans cannot possibly bring about an incipient speciation, for sperm whales around the entire globe remain reproductively integrated. It remains a mystery whether the clans of their natal units have any influence at all on the socializing that takes place between male sperm whales.194
Other differences are apparent at the oceanic level. Sperm whale units in the Atlantic are smaller: on average seven adults, most often of a single matriline. In the Pacific, they are composed of around eleven adults, usually made up of two distinct matrilines. Pacific units are also more likely to join with others into larger groups. They are typically spotted in groups with a mean of twenty-eight individuals, meaning two or three separate family units together.195 This is intriguing when analyzed alongside the fact that in the Pacific, different clans are constantly overlapping with each other, while they only rarely encounter each other in the Atlantic. The dynamics of “us and them” shape daily existence for Pacific sperm whales, structuring their associations beyond their immediate kin, in a manner that is simply not the case for their Atlantic counterparts. An interesting binary emerges between the two ocean basins: in the Atlantic, the social structure seems to be a more organic outgrowth of matrilineality—the units all belong to clans, but it infrequently determines social decisions that they make. But in the Pacific, the social structure seems much more thoroughly institutionalized. Individuals are embedded in close relationships with non-relatives as part of the same unit. They associate more frequently with other units, in multicultural settings where clan affiliation is fundamental. And at the same time as they have increased cross-clan encounters, the coda repertoires of Pacific clans are substantially more divergent from one another than those in the Atlantic.196 This strongly suggests schismogenesis as a force shaping sperm whale vocal dialects—and perhaps other as-yet unobservable behaviors.
When researchers measure the frequency of different codas clicked by sperm whales, three types predominate across all oceans. The most common is of course the coda signaling their clan, announcing their vocal dialect and social identity. The second most common is a coda that identifies that whale individually. Usually this is five clicks with a subtly different spacing between them, and each whale has a unique one that they will use to announce themselves. And the third most common coda type is one that all members of a given family unit share.197 This statistical analysis of sperm whale codas gives the unambiguous impression that as individual whales are clicking their way through the ocean, they are announcing to everyone around to hear their individual name, their family name, and the nation to which they belong. As there are far more codas that we have recorded that are not associated with individual or group identity, there is a great deal of other information that sperm whales seem to communicate to one another with them, often over great distances.
Adults can be immediately summoned to the surface by calls for help if babies are in danger. Certain killer whale populations pose a serious threat to sperm whales, and researchers have witnessed sperm whale families under attack by them summon dozens of other sperm whales from miles around, including lone males, to their collective defense. In one such episode, an attack by a single killer whale female on a sperm whale unit drew a second nearby unit, then twenty whales, then thirty, then an estimated fifty whales, formed into a massive wall shielding the young—the young of complete strangers, no less—from the attacker.198 We have numerous accounts from whalers of their attacks on a family of females and babies summoning massive males, who—at least in the earlier days of relatively small, wooden vessels—would attack and attempt to sink the whaling ship. These whalers suspected that they were capable of sounding long-distance summons to one another. Analysis of historical whaling records also strongly suggests that sperm whales learned and taught one another effective avoidance strategies for whaling ships that suddenly cut their kill rates by more than half.199
With our painfully recent knowledge of sperm whale clans, much of previously documented sperm whale behavior appears in an entirely different light. For example: we know that before the rise of industrial whaling in the late eighteenth century, sperm whales lived in small matrilinear family groups of at most one to two dozen. But then during those two hundred years of intensive hunting, they radically changed their social structure, gathering into enormous herds of hundreds of individuals. I now find this passage from Herman Melville’s novel Moby-Dick to be chilling:
Owing to the unwearied activity with which of late they have been hunted over all four oceans, the Sperm Whales, instead of almost invariably sailing in small detached companies, as in former times, are now frequently met with in extensive herds, sometimes embracing so great a multitude, that it would almost seem as if numerous nations of them had sworn solemn league and covenant for mutual assistance and protection… Even in the best cruising grounds, you may now sometimes sail for weeks and months together, without being greeted by a single spout; and then be suddenly saluted by what sometimes seems thousands on thousands.200
When sperm whales were hunted in this way, humans did not even know they made sounds (much less the loudest, most powerful sounds of any living thing), referring to them instead as “silent giants.” Knowledge of their vocal clans was, at that time, impossible. Perhaps, then, Melville’s poetic choice of the word “nations” here was rather more apt than he could have ever understood.201
Such threats to sperm whales may explain the mystery of societal differences between ocean basins. While the sail- and row-powered whaling operations of the eighteenth and nineteenth centuries slaughtered them across every ocean, the floating factories armed with harpoon guns and petroleum engines that exterminated millions of whales in the twentieth largely spared the sperm whale populations of the western North Atlantic. The Pacific sperm whales, on the other hand, were killed in huge numbers between 1950 and 1986.202 This is still living memory for many of the survivors. It is hypothesized that larger, more institutionalized social structures in the Pacific were a defensive response to this violence. Another differential threat may also have impacted the relative importance of clans, over a longer period: killer whales. The killer whale cultures that are interested in hunting sperm whales are completely absent from the North Atlantic, but have a presence in the Pacific, and Pacific sperm whales respond quickly with collective defensive formations when these predators appear.203 “[I]n the Atlantic,” by contrast, Whitehead and Rendell write, “we have seen killer whales cruise right through a group of foraging sperms without any obvious reaction by the other species.”204 The social impact of these threats amounts to a cultural interplay between killer whales and sperm whales, not to mention between humans and sperm whales. One’s socially transmitted specialization in attacking sperm whales requires an adaptive cultural change in sperm whale social forms. And indeed, their past massive groups that Melville wrote of disappeared along with that era’s whalers.205 What matters for understanding sperm whale societies is not simply their biology but also their history.
Another perplexing illustration of sperm whales as a historical species comes from long-term research in the Galapagos. Between 1985 and 1999, scientists documented the presence of sperm whales belonging to the Regular and Plus-One clans in the waters around the islands. Their presence there declined over the course of the nineties, and after 2000 they had disappeared completely, so the researchers moved on. Following reports of renewed sightings more than a decade later, they returned to find hundreds of sperm whales. But these belonged to two entirely different clans, which had not been previously documented in that area: the Short and Four-Plus clans.206 A stunning, rapid cultural turnover had taken place. Rendell, one of those researchers and a co-author of the paper discussing, remarked, “We don’t understand what drives these processes. If they were human societies, you might call it history.”207
Scientific research into animal culture is painfully short lived. We have assembled merely a snapshot of the distribution of cultural difference—and a fragmentary snapshot at that. Enormous questions remain, which could quite plausibly require centuries of further research to begin to answer. How does new identity formation of sperm whale clans actually take place? What are the mechanisms of their ethnogenesis? How are the existing communities and clans related to one another historically? What else are they able to say to one another with their codas? We will continue to observe them in long term studies in the hopes of witnessing such cultural fission events take place in real time. Perhaps there may be certain scientific breakthroughs in understanding how new codas form, such that we could piece together how existing ones were related to each other historically, as historical linguists are able to reconstruct ancestral human languages. What we may rest assured of is that extraordinary unknowns dance just out of sight, beneath the surface of the waves.
* * *
Social learning is widespread throughout the animal kingdom, but it appears to be a necessary yet insufficient condition for the evolution of societies, of second natures. Bookchin wrote in Remaking Society that,
The human socialization process from which society emerges—be it in the form of families, bands, tribes, or more complex types of human intercourse—has its source in parental relationships, particularly mother and child bonding. The biological mother, to be sure, can be replaced in this process by many surrogates, including fathers, relatives, or, for that matter, all members of a community. It is when social parents and social siblings—that is, the human community that surrounds the young—begin to participate in a system of care, that is ordinarily undertaken by biological parents, that society begins to truly come into its own.208
This, as should be clear by now, also is true of the mother-centric societies of elephants, killer whales, and sperm whales in very similar ways. Though many other thinkers have focused on attributes like intelligence and material culture, Bookchin’s identification of second nature’s biological roots with the complexity of structures of care for children is, in my view, a crucial insight. It is also related, evolutionarily speaking, to their drawn out periods of juvenile development. Extended socialization of one generation by those before it may be a prerequisite for highly developed cultures and social institutions. That prerequisite is itself accompanied by a set of necessary preadaptations. Reproductive strategies that are numbers games, producing huge numbers of eggs in the hopes that a few survive, are non-starters. Active parental investment in the process of a baby becoming a mature adult is essential. It would seem, in other words, that one must be either a mammal or a bird to set in motion cultural development.
Other features, like living in groups, impose selective pressures for intelligence and social learning. Social environments where young individuals can learn behaviors from conspecifics other than their own parents create pathways for far more dynamic processes of social transmission. Cooperation, when necessitated by optimal available foraging strategies, predator defense, and the demands of raising offspring, opens the adaptive space for innovating collective behaviors. These, crucially, are the behavioral substrate of concepts of identity and belonging, of an “us” with whom cooperation is advantageous and a “them” with whom it is risky. Different ecologies and physical environments also play important roles: those characterized by steady variation over time advantaging social learning and group interdependence the most.
In breaking down the evolutionary pressures opening and confining the possibilities for culture, we can see simultaneously why animal societies like those of elephants and toothed whales are uncommon and why they would emerge independently through convergent evolution, time and again. We can expect that they will continue to do so, in the millions of years to come.
Animal Cultures and Conservation
In 2007, Japanese whale biologist Toshio Kasuya—the discoverer of (among other things) menopause in short-finned pilot whales and a vocal critic of the Japanese government’s support for “scientific whaling”—received a lifetime achievement award from the Society for Marine Mammalogy. In his acceptance speech, he discussed the importance of culture in the lives of cetaceans and made the case that conservation biology must consider nonhuman cultural diversity as an aspect of biodiversity. Failure to protect unique cultural communities of animals in addition to species, he explained, would damage the adaptiveness of species in the face of environmental change.209
Other conservation biology institutions have embraced these ideas, at least in principle. In 2014, the Scientific Council of the Convention on the Conservation of Migratory Wild Animals (CMS) assembled a report on “the extensive range of circumstances in which social structure, social learning, and cultural variation in whales and dolphins can affect the planning or outcomes of conservation efforts,” leading to the adoption of a historic resolution in which the treaty’s signatories “formally acknowledged the importance of social learning and culture for the conservation of some highly social species.”210 The respective marine fishery agencies of both the Canadian and US governments finally recognized the northern and southern resident killer whales as culturally distinct populations that needed to be independently protected in the mid-2000s, following campaigning and court battles led by scientists and whale advocates.
We can consider the conservation significance of animal cultures from two angles: viewing animal culture as a variable that impacts conservation success and failure, and viewing animal culture as an object of conservation in itself. Both of these are of key concern for social ecology: the former as a condition of an effective response to biodiversity crisis amid catastrophic planetary changes, and the latter as a way to bring social ecology’s deeper and more theoretically developed conception of diversity to bear upon ecological practice. One is a matter of good science, the other of good philosophy.
Some of the conservation implications of animal cultures are by this point likely obvious. Culture, as we have explored, shapes and contributes to biological fitness and accelerates the possible pace of behavioral adaptation. It enmeshes the survival of individuals in social processes that human interventions are unlikely to be able to reconstruct—though we may easily destroy them. Culture creates resiliences—as in the socially transmitted knowledge of elephant matriarchs that helps them find water for their families during drought—and it creates vulnerabilities, as with the southern resident killer whales’ persistent commitment to the lifeways of their ancestors despite precipitous declines in the abundance of Chinook salmon in the Puget Sound.
To unpack these implications a bit further, I see the following as key principles of a conservation biology informed by animal culture:
- Cultural animals are not interchangeable representatives of their species.
Conservation biology has historically and conventionally understood species as populations. Individual animals are assessed as fractional components of their species. Animal cultures, however, reveal the complete inadequacy of this mode of thought. Not all animal subpopulations are or behave the same. The threats faced and the conservation responses required by culturally distinct groups of the same species may be highly differentiated. Their cultures may reproductively isolate them from each other by language (an inability to communicate across cultural divides), by ritualized behaviors around mating, or by markers of identity, shrinking down the population units of conservation interest.
Furthermore, some knowledgeable individuals play outsized roles shaping the survival potential of their cultural subpopulation and their species as a whole, well beyond their numerical contribution to its headcount. Ivory hunters, for instance, target the individual elephants with the largest tusks—the oldest individuals with the most socially accumulated knowledge, and with the most important social function in guiding younger generations and transmitting vital cultural information. Extermination of large bulls has brought social chaos into the male social world. The loss of wise matriarchs can spell death for entire families. “If groups rely on older members for their store of social knowledge,” Karen McComb and her elephant researcher colleagues write, “then whole populations may be affected by the removal of a few key individuals.”211 With the targeted destruction of key culture-bearers has come the destruction of their social institutions.
- Cultural animals rely on social continuity for survival.
This fact collides most painfully with actually practiced conservation when it comes to captive breeding and reintroduction programs. Reintroduced animals with no relationship to their wild forebears have no access to the behavioral traditions and knowledges developed by their ancestors, upon which survival often depends. This is one of the primary reasons so many reintroduction programs are failures. For animals that depend on social learning for survival, extinction in the wild is simply extinction, with the exception of the limited cases where humans can both know and be the transmitters of vital animal knowledge (such as efforts to teach captive-bred whooping cranes their ancestral migratory routes by leading them on these journeys with ultralight aircraft). If captive breeding and release for cultural animals is to succeed, it will necessarily rely on some form of partnership with culturally knowledgeable animals to bridge social discontinuity.
Extermination of large numbers of a given animal species may also sever social continuity, even if the species is itself not yet extinct in the wild. Conservation properly informed by science cannot solely aim for the preservation of genes but also of social knowledge of community lifeways and the conditions of its transfer: a minimum threshold of stability and freedom in those animals’ own lands and waters.
- Cultural loss for nonhumans is permanent.
No animals but human beings possess archives. Animal cultures are stored nowhere but in animal minds and disappear when those animals are deprived of the ability to pass them on before their deaths. The pressures capitalism and other societies of domination have imposed on still extant cultural species have already done irreparable forms of harm to them. For animals like blue whales and African elephants that have lost upwards of 99% of their population, immense troves of knowledge have been destroyed forever, and the survivors have been radically transformed by their species apocalypses. Our orientation towards these animals cannot be restoration per se, but opening the ecological space for survivor remnants to build new cultures through their recovery. The cultures that have been lost were the result of millions of years of cultural development and change, and anything resembling cultural recovery will require many of their generations. Even in this best case scenario, their trajectories are forever changed.
What this also means is that, in an age of biodiversity collapse, any scrap of socially learnable animal knowledge or behavioral idiosyncrasy that can be protected in even very small populations is a treasure for the future, with the potential to enable whole trajectories of their ecological and social existence in coming millennia.
- Cultural animals are agents of their own futures.
No matter how deeply we study ecology and behavior, it is impossible for us to predict the behavioral innovations that animals will develop in response to conditions changing all around them. The future of a threatened species of cultural animal rests, to varying but always present degrees, on the ingenuity, decision-making, and relationships of the animals themselves. They are not and cannot be managed as merely objects of conservation that we act upon. They are also our partners in the project of their recovery, and their own adaptations are the most powerful tool in that project’s arsenal. Operating accordingly requires us to undertake a radical conceptual shift about the agency of animals and the intersubjectivity at work when we attempt to help them. They will, inevitably, surprise us.
***
From the standpoint of social ecology, animal cultural variation would not be simply a factor shaping the conditions for biodiversity and species survival. It is, in itself, an expression of biological diversity, an emergence of life’s inherent drives toward differentiation, a foundation for biotic stability and flourishing. The same philosophical principles that push social ecologists to embrace ecological diversity and human cultural diversity extend towards the diverse cultural repertoires of nonhuman animals that enrich our shared world. From the ethical standpoint of dialectical naturalism, which grounds ethical principles in the developmental tendencies of nature itself, animal cultural diversity must be defended—and indeed, propagated—with the same urgency as species and ecosystem diversity.
Conclusion: An Interspecies Third Nature
What, ultimately, does any of this mean for social ecology’s philosophy or political project? And how might social ecology’s conceptual framework help illuminate the evolutionary emergence of animal cultures and societies? It is my view that the paradigm shift taking place around animal culture is a philosophical gift rather than a philosophical threat to social ecology. These are phenomena that social ecology has conceptual tools ready-made to make sense of and to theorize. But to do so will first require a self-corrective response.
As explained in my introduction, I believe that Bookchin’s outbursts of anthropocentrism were motivated by two fears: the fear of misanthropic ecologists minimizing the value of humanity and the fear of defenders of class society naturalizing hierarchy as a biological fact of human beings. Both fears are justified, yet sit upon a lack of comprehension of the complexity and variability of animal societies. Blurring the “ontological divide” between humans and other animals, we can now say with real confidence, need not anchor us to any particular social forms as biological destiny. Many of these discoveries came in Bookchin’s final years of life, however, when his focus had narrowed to political questions, the cutting edge of ecological and evolutionary science largely displaced to the peripheries of his active thinking. Other discoveries were made or became public only after his passing.
Even so, the result is that Bookchin’s ecological philosophy is hobbled by one truly staggering contradiction, which if wrestled with in any seriousness ought to have led him to anticipate rather than dismiss findings about the expansive possibilities of nonhuman social life.Consider social ecology’s account of the evolution of subjectivity and freedom. At its simplest, a nascent subjectivity is present in even a unicellular organism that can react to particular stimuli and environmental conditions. Evolutionarily, however, such programmed reaction can only get a living thing so far. Intelligence in various forms has therefore evolved repeatedly and independently, such that organisms in a dangerous and dynamic world can approach the problems of survival with a higher order of flexibility in their responses and decisions. Its key mechanism is learning, the retention of past experience for the honing of future behavior. The animal mind is an evolutionary answer to a world of complexity, where preprogrammed responses are inadequate to the task of survival. This basic principle is also at work in the social lives of animal communities, expanding the space of biological possibility for freedom. Some herds, schools, and colonies are indeed essentially locked in a given model of collective existence, baked immutably into their biology. But this is simply not as resilient to shifting circumstance as the ability to adapt collective behaviors and the social form itself to the species’ needs. And so mutable societies have also emerged again and again, most concentrated among the animal kingdom’s most social and intelligent species. The tendency towards the independent emergence of multiple second natures is a consequence of natural evolution itself.
From a different angle, a social ecologist might interpret animal culture as an embodiment of the principles we see throughout nature and the interactions between them. The scientific literature on social learning places most emphasis on behavior copying: attempting to unpack how precisely one animal’s behavior is adopted by another. But, Safina points out, this can only be one side of the coin for culture. A given animal species must have the ability to imitate and to innovate, to be (perhaps in different individuals at different times) simultaneously conformist and avant-garde. It is the interplay between both of these that makes culture possible.212 Parallel principles are articulated in Bookchin’s ecological philosophy, such as “spontaneity”: a viable stand-in for innovation. Adoption of our fellows’ learned behaviors is at root a variety of mutualism, another tendency Bookchin saw running through nature. Or we might theorize the evolution of culture as emerging from the interaction between subjectivity and sociality. Dialectical naturalism points to a real historical development in the self-directivity of life as a product of such interactions over deep time.
This philosophical outlook is why Bookchin did not see humanity as a historical accident or aberration, but as an embodiment of inherent natural tendencies emerging from the process of evolution towards greater levels of consciousness. This way of thinking about nature, however, lies most uncomfortably with Bookchin’s other claims about an ontological divide lying between us and other animals. If our most remarkable capacities for a social remaking of ourselves and how we exist in the world are the results of tendencies running through the whole history of life back to the first steps across the Darwinian threshold, then we would expect such capacities to emerge repeatedly and independently among a diverse array of living beings. Instead, Bookchin hunkered down around the notion that the emergence of society was a fluke, a single instance of evolutionary luck accompanying the rise of but one species. His human exceptionalism runs counter to the whole thrust of his broader nature philosophy. It is in the interests of social ecology’s own internal coherence that Bookchin’s limiting dogmas on nonhuman animals must be shed.
Jane Goodall once wrote that “It is important that science dares to ask questions outside the prison of the biased mind, dares to explore new areas of animal being.”213 Social ecology possesses invaluable philosophical resources to be at the leading edge of that exploration. Its existing canon concerning these subjects, however, trails conservatively among the tired doctrines of a more ignorant age.
If social ecology could accommodate a reassessment of “second nature” to encompass the cultural lifeworlds of various animal species, what would this mean for its political project? More scientifically grounded and visionary wildlife conservation practices are essential but merely scratch the surface of potential implications. Here I request a certain concluding indulgence in the utopian imagination, for we are treading deep into the realm of the speculative.
Bookchin imagined that social revolution could realize
…the potentialities of humanity to develop, however falteringly, mature, self-conscious, free, and ecological communities. I call this integration of the best in first or “biological” nature and second or “social” nature an emergently new “third” or free nature—that is, an ethical, humanly scaled community that establishes a creative interaction with its natural environment. From a processual standpoint, I refuse to bifurcate that continuum we call “Nature” into a biological world and a social world that stand in flat opposition to each other. Both are in a very real sense natural, and their naturalness finds its evolutionary realization in those remarkable primates called human beings who, consciously responding to a sense of obligation to the ecological integrity of the planet, bring their rational, communicative, richly social, imaginative, and aesthetic capacities to the service of the nonhuman world as well as the human.214
This third nature would entail creative human participation in the future course of evolution, stewarding not only our ecosystems but a rationally guided unfolding of natural history.
However, if second nature is reconceptualized to be plural rather than singularly human, that would point to a reconceptualization of third nature as well. What I want to suggest is that a new dialectical unfolding emergent from second nature might not be a matter of humanity shepherding nature’s development but of a dialogic collaborative relationship between second natures that changes the course of life’s history on our planet. Short of extraterrestrial contact, there are no exchanges between minds that could inject as radical a shift in human thinking as this. Do we have the imagination beyond the prison of what exists to see a place in the world commune of communes for other animals—not only to be considered but to speak and to decide?
Since 2020, an interdisciplinary team of sperm whale biologists, robotics engineers, and machine learning specialists have been working to build an immense dataset of audio recordings of codas and metadata about the context of their usage, for artificial intelligence tools to attempt to decode. In extraordinary ways, new technologies have and will continue to radically expand the world of sensory information we can detect and analyze, from chemical signatures to infrasound to wavelengths of light outside the visible spectrum. Projects of interspecies “translation” may of course still ultimately be futile. They may even be impossible in principle. But we may be also on the cusp of a transition in how we think about our relationships with non-humans, where the ability to talk to animals is shifting from the stuff of fantasy to the stuff of science fiction, and perhaps eventually into the stuff of political theory.
Bookchin believed that human potential lay in its ability to become “nature rendered self-conscious and self-reflexive.” Thankfully, when we wield that consciousness to look around us, we do not find ourselves alone.
- Murray Bookchin (1979), “The Power to Create, the Power to Destroy,” Towards an Ecological Society.
- As an excellent but lonely example, see Dayton Martindale’s contribution to an earlier issue of Harbinger, “The Social Ecological Case for Animal Liberation: Towards an Interspecies Communalism” (2019). https://harbinger-journal.com/issue-1/the-social-ecological-case-for-animal-liberation/.
- Murray Bookchin, Re-enchanting Humanity: A Defense of the Human Spirit Against Antihumanism, Misanthropy, Mysticism, and Primitivism (Cassel Press, 1995), 101.
- Murray Bookchin, Remaking Society: A New Ecological Politics (Oakland, AK Press, 2023), 23.
- Different accounts of the developmental tendencies of nature appear in Bookchin’s work, including “complexity, specialization, and consciousness” (in The Philosophy of Social Ecology), and elsewhere as “diversity,” “freedom,” “creativity,” “spontaneity,” “mutualism,” “self-organization,” and/or “complementarity.” (See Murray Bookchin, “The Transition to the Ecological Society: An Interview by Takis Fotopoulos,” Institute for Social Ecology, 11 Sept. 1992, https://social-ecology.org/wp/1992/09/the-transition-to-the-ecological-society-an-interview-by-takis-fotopoulos/ [Originally published in Society and Nature 1(3)]; and Brian Morris, “Remembering Murray Bookchin (1921-2006): Dialectical Naturalism,” Anarchist Studies 30(2), https://journals.lwbooks.co.uk/anarchiststudies/vol-30-issue-2/article-9600/). The language of “differentiation, interdependence, and subjectivity” is my own, which I consider to be more precise and scientifically defensible, elaborated during my talk at the 2023 Institute for Social Ecology summer intensive course. https://youtu.be/MfYTxLuomTE?si=00PZFO4H60eddYFP.
- In Bookchin’s earlier work, prior to his embrace of “first nature and second nature,” he described these same concepts as “nature and society.”
- As we will see later, the exact same problem emerges in the debates between anthropologists and ethologists about the meaning of the word “culture.” Anthropologists typically list out features of human cultures, because they take as a given that culture exists for humans and humans alone. But scientists studying animal behavior and social learning require a rigorous definition against which they can test their observations and around which to develop experiments. That has led to a deeper theorization of what culture is.
- Murray Bookchin, “What Is Social Ecology?,” in Social Ecology and Communalism (Oakland, AK Press, 2006), 29. The essay was originally published in an anthology edited by Michael Zimmerman, Environmental Philosophy: From Animal Rights to Radical Ecology (Englewood Cliffs, N.J.: Prentice Hall, 1993), although it was revised both in 1996 and 2001. In a recorded lecture from 1996, Bookchin stated that “[H]uman beings are constituted by natural evolution itself to intervene in the environment. Not simply to adapt to it. Not simply to find a niche in the environment or secure way of living. But also to change it. That’s what is unique about human beings: using their rationality, such that the environment is suitable for them; not leaving it up to evolution to make them suitable to changes in the environment. Now that’s an enormous qualitative step. It’s a qualitative difference.”
- Karl Marx, Capital: A Critique of Political Economy, Vol. I, trans. Ben Fowkes (New York, Penguin books, 1976), 284.
- This matter of “technical foresight” is also a question largely kept open by goalpost-moving to disregard each new wave of findings on the extent of non-human cognition.
- Bookchin, “What Is Social Ecology?,” 30.
- Murray Bookchin, “Second Nature, Part 1 (1996).” https://www.youtube.com/watch?v=F_6WsYBMow4. Bookchin elaborates how he uses the word “institution”: “[A] way of interacting in a mutable way. You can change institutions.”
- Bookchin, Remaking Society, 21.
- ibid 19-20.
- Peter J. Richerson and Robert Boyd, Not by Genes Alone: How Culture Transformed Human Evolution (Chicago and London, University of Chicago Press, 2005), 7.
- John Maynard Smith, Evolutionary Genetics, Second Edition (Oxford, Oxford University Press, 1999), 11.
- Peter Medawar, “Unnatural Science,” New York Review, February 3, 1977. https://www.nybooks.com/articles/1977/02/03/unnatural-science/. Also published in The Strange Case of the Spotted Mouse and Other Classic Essays on Science (Oxford, Oxford University Press, 1977), 144-161. In this essay, he continues: “Apart from being mediated through nongenetic channels, cultural inheritance is categorically distinguished from biological inheritance by being Lamarckian in character; that is to say, by the fact that what is learned in one generation may become part of the inheritance of the next. This differentiates our characteristically human heredity absolutely from ordinary biological heredity…” Medawar further elaborates these ideas in his essay “Technology and Evolution” (http://bactra.org/Medawar/technology-and-evolution/).
- Andrew Whiten, “The Burgeoning Reach of Animal Culture”, Science (Vol. 372, No. 6537, April 2021). https://www.science.org/doi/10.1126/science.abe6514.
- Richard Dawkins, The Selfish Gene (Oxford, Oxford University Press, 1976).
- I myself am oversimplifying the biological side here, as genetic material can also be transmitted horizontally: especially among archaea and bacteria, but not exclusively. Horizontal gene transfer is also documented from bacteria to plants, fungi, and animals, as well as between plants, fungi, and animals. These transfer events are not accounted for in the modern synthesis (Darwinian natural selection, Mendelian genetics, and DNA), and complicate the Darwinian account of the history of life. See David Quammen, The Tangled Tree: A Radical New History of Life (New York, Simon & Schuster, 2019).
- Luigi L. CavalliI-Sforza and Marcus W. Feldman, Cultural Transmission and Evolution: A Quantitative Approach (Princeton, Princeton University Press, 1981).
- This fact is one of the limitations of “dual inheritance” as a framing: it’s not all inheritance!
- See Murray Bookchin, The Philosophy of Social Ecology (Montreal and New York, Black Rose Books, 1996), 77-96.
- See James C. Scott, Against the Grain: A Deep History of the Earliest States (New Haven and London, Yale University Press, 2017), 37-43.
- John Maynard Smith, Evolutionary Genetics, second edition (Oxford, Oxford University Press, 1999), 11.
- Cecilia M. Heyes, “Social Learning in Animals: Categories and Mechanisms,” Biological Reviews of the Cambridge Philosophical Society (Vol. 69, No. 2, 1994), 207-231.
- The primary maladaptive danger of social learning is the problem of information parasitism. If, for instance, all members of a species simply copy the behaviors of others, they will be collectively trapped in a circular dead end with behaviors that may not actually work. Knowing who to copy and how to test socially learned behaviors through environmental interaction is important for this capacity to be properly adaptive.
- Carl Safina, Becoming Wild: How Animal Cultures Raise Families, Create Beauty, and Achieve Peace (New York, Henry Holt and Company, 2020), 46.
- Aristotle, History of Animals, 83. https://www.loebclassics.com/view/aristotle-history_animals/1965/pb_LCL438.83.xml.
- Charles Darwin, The Descent of Man, and Selection in Relation to Sex (New York, Penguin Classics, 2004), 161. It is worth noting that Darwin has been repeatedly criticized by subsequent generations of biologists for over-imagining the intelligence and social learning capacity of various nonhuman animals. His views are only now being vindicated by more recent scientific research. Peter Richerson and Robert Boyd write in Not by Genes Alone (2005, 16), “From the biologists’ point of view, Darwin’s belief in the inheritance of acquired variation was his greatest error. Darwin thought ‘inherited habits,’ by which he meant something very close to human culture, were important in a wide variety of species. In a sense he was correct—simple forms of social learning are widespread in the animal kingdom. However, Darwin imagined that even honeybees had humanlike imitative capacities, whereas the best modern evidence, as we shall see, suggests that all other animals, including our closest ape relatives, have rudimentary capacities for culture compared with ourselves.” Richerson and Boyd have since tempered this claim of human uniqueness. (Personal correspondence with Richerson, January 31, 2024. See also Natalie Angier, “Meet the Other Social Influencers of the Animal Kingdom,” The New York Times [May 7, 2021]. https://www.nytimes.com/2021/05/07/science/animals-chimps-whales-culture.html.)
- In their introduction to The Question of Animal Culture, Kevin Laland and Bennett Galef note a sort of behavioral Lamarckianism independently reached by various late nineteenth-century theorists of evolution (C. L. Morgan, James Mark Baldwin, Douglas Spalding, and Henry Osborn), who each “suggested that organisms could survive ecological challenges by virtue of their acquired knowledge and skills, frequently learned from others, and that this would then channel natural selection to favor unlearned versions of the same adaptive behavior.” Kevin N. Laland and Bennett G. Galef, “Introduction” in The Question of Animal Culture (eds. Kevin N. Laland and Bennett G. Galef, Cambridge, Massachusetts and London, Harvard University Press, 2009), 2-3. Citing C. L. Morgan (1896), Baldwin (1896), Spalding (1873), and Osborne (1896).
- James Fisher and R. A. Hinde, “The Opening of Milk Bottles by Birds,” British Birds, 1949.
- William H. Thorpe, “The Process of Song-Learning in the Chaffinch as Studied by Means of the Sound Spectrograph”, Nature (Vol. 173, 1954), 465-46; William H. Thorpe, “Further Studies on the Process of Song Learning in the Chaffinch (Fringilla Coelebs Gengleri)”, Nature (Vol. 182, 1958), 554-557; Peter Marler and M. Tamura, “Song Dialects in Three Populations of White-Crowned Sparrows”, Condor (Vol. 64), 368-377.
- Birds have remained an important area of study because of the ease with which researchers can build experiments to test vertically transmitted social learning, based on two key bird characteristics: they hatch from eggs, and so can be readily cross-fostered, and many species imprint at birth onto the first thing they see, enabling highly flexible manipulation of who and what young birds learn from.
- Imanishi was a fascinating figure, whose work is of real philosophical significance for social ecology. The primary influences on his novel thinking about evolution were Buddhist philosophy and Peter Kropotkin’s Mutual Aid: A Factor in Evolution, leading to a body of ideas that overlap closely with Bookchin’s in seeing evolution as a process animals participate in.
- Japanese macaques also exhibit a range of other socially learned behaviors, such as eating fish, removing chaff from wheat, bathing in hot springs, and a strange and arbitrary practice called “stone handling”—the latter of which we have also documented spreading through a population in real time. See Frans de Waal, The Ape and the Sushi Master (New York, Basic Books, 2001); Susan Perry, “Are non-human primates likely to exhibit cultural capacities like those of humans?” In The Question of Animal Culture (2009), 247–68; and Michael A. Huffman, “Stone-play of Macaca fuscata in Arashiyama B troop: transmission of a non-adaptive behavior,” Journal of Human Evolution (Vol. 13, No. 8, 1984), 725–35; and Michael A. Huffman, “Acquisition of innovative cultural behaviors in nonhuman primates: a case study of stone handling, a socially transmitted behavior in Japanese macaques,” In Social Learning in Animals, ed. by C. M. Heyes and B. G. Galef (San Diego, Academic Press, 1996), 267–89
- Andrew Whiten, “The Burgeoning Reach of Animal Culture,” Science (Vol. 372, No. 6537, April 2021). https://www.science.org/doi/10.1126/science.abe6514.
- I cursorily overview five social learning mechanisms, but other breakdowns include even further hairsplitting. Michael Tomasello lists “social facilitation,” “response facilitation,” “matched dependent learning,” “response matching,” “local enhancement,” “stimulus enhancement,” “mimicking,” “emulation learning,” “action-outcome contingency learning,” “imitation,” “program-level imitation,” “movement imitation,” and “imitative learning,” all with fine-grained distinctions (in “The Question of Chimpanzee Culture, Plus Postscript [Chimpanzee Culture, 2009],” in The Question of Animal Culture [2009], 199).
- It is possible to learn through stimulus enhancement without even witnessing the other individual perform the behavior directly. For instance, if a bird comes across a bottle of milk whose lid has been punctured by a beak, they can learn a possible way to access what is in other similar bottles, even with the inventive other bird being physically absent. (This is why the “or its products” part of Heyes’ definition is important.) Examples of local and stimulus enhancement have been identified in, among many others, great tits, common eastern bumblebees, and wood frog tadpoles. See John R. Krebs, Michael H. MacRoberts, and J.M. Cullen, “Flocking and Feeding in the Great Tit Parus major — An Experimental Study,” Ibis: International Journal of Avian Science (Vol. 114, No. 4, 1972), 507-530; Bradley D. Worden and Daniel R. Papaj, “Flower Choice Copying in Bumblebees,” Biology Letters (Vol. 1, No. 4, 2005), 504-507; and Trevor L. Chapman et al., “A Test of Local Enhancement in Amphibians,” Ethology: International Journal of Behavioural Biology (Vol. 121, No. 3, 2015), 308-314.
- In comparative studies of human children and chimpanzees, a demonstrator will show each how to perform a novel task, but inserts multiple pointless steps into it. Typically, chimpanzee subjects ascertained the goal and determined which steps were necessary towards that end, skipping over the superfluous ones. Human children, however, dutifully perform each of the demonstrator’s steps no matter how inefficient or obviously irrelevant to the goal at hand. One way of framing these results, as primatologist Frans de Waal has, is that chimpanzees are actually more intelligently scrutinizing problem solvers than human children are. But for our purposes, what this signals is that chimpanzees are highly capable emulators, while humans, or at least human children, are relying on imitation. This has significant implications for each species’ potential for cultural development. See Nagell, Olguin, Tomasello, 1993, “Processes of social learning in the tool use of chimpanzees (Pan troglodytes) and human children (Homo sapiens),” Journal of Comparative Psychology, 107, 174-186; and Frans de Waal, Are We Smart Enough to Know How Smart Animals Are? (New York, W.W. Norton and Company, 2016). See also Michael Tomasello, et al, “Observational learning of tool use by young chimpanzees and enculturated chimpanzees,” Human Evolution (Vol. 2, 1987), 175-183. This experiment showed that chimpanzees learned strategies for how to pull food into reach from each other, but that they did not precisely imitate how they did it.
- This is an excellent illustrative example of some of the evolutionary tradeoffs of social learning. There are real tensions between adopting whatever behavior you see from others and engaging in your own problem-solving process through interaction with your environment, with imitation possibly facilitating the spread and maintenance of maladaptive behaviors—despite the greater potential for cultural development that it unlocks.
- See T.M. Caro and M.D. Hauser, “Is There Teaching in Nonhuman Animals?,” The Quarterly Review of Biology (Vol. 67, No. 2, 1992).
- Other perhaps more surprising examples of teaching in animals are meerkats, who develop their young’s scorpion-hunting skills by giving them practice prey with the stingers removed; pied babblers, birds of southern Africa who as parents use stimulus-response conditioning to teach their young to associate a particular call with food; and cheetahs who, similarly to meerkats, present their young with partially disabled prey to teach them to hunt. See Alex Thornton and Katherine Mcauliffe, “Teaching in Wild Meerkats,” Science (Vol. 313, No. 5784, 2006), 227-9; Nichola H. Raihani and Amanda R. Ridley, “Experimental evidence for teaching in wild pied babblers,” Animal Behaviour (Vol. 75, No. 1, 2008), 3-11; and Laurie Marker, “Cheetahs Race for Survival: Ecology and Conservation,” chapter 4 in Wildlife Population Monitoring, ed. Marco Ferretti (IntechOpen, 2019), 53-72. While direct evidence for teaching does not yet exist for my third case species (sperm whales), this is likely due to the limits on our ability to observe them—researchers believe teaching behavior is indeed highly likely. See Ana Eguiguren, Christine M. Konrad Clarke, and Mauricio Cantor, “Sperm Whale Reproductive Strategies: Current Knowledge and Future Directions,” chapter 19 in Sex in Cetaceans: Morphology, Behavior, and the Evolution of Sexual Strategies, eds. Bernd Würsig and Dara N. Orbach (Springer Cham, 2023), 443-467.
- Edward B. Tylor, Primitive Culture: Researches into the Development of Mythology, Philosophy, Religion, Language, Art, and Customs (London, Cambridge University Press, 1871), preface. As with Bookchin’s account of second nature, there is a conceptual link between assumptions about culture being singularly human and its definition in terms of a list of characteristics understood as cultural. Interrogating the culture concept without those assumptions facilitates a deeper theorization of what exactly unites those various cultural characteristics. For an anthropologist’s scathing take on even the possibility of culture among nonhumans, see Tim Ingold, “The Use and Abuse of Ethnography,” Behavioral and Brain Sciences (Vol. 24, 2001), 337.
- In Whiten’s original graphic, he also includes “social information transfer” as a layer beneath “social learning.” Social information transfer refers to some kind of information received from another animal that impacts behavior but not in any persistent way. His example is of a bumblebee drawn to a given patch of wildflowers because it sees other bees heading there. This is social information transfer, which only becomes social learning if the bee actually learns something from it: namely, if it returns to that patch of wildflowers in the future (regardless of the presence of other bees) because it has learned that it is a good place to go.
- Etienne Danchin et al., “Cultural Flies: Conformist Social Learning in Fruitflies Predicts Long-Lasting Mate-Choice Traditions,” Science (Vol. 362, No. 6418, 2018), 1025-1030.
- See Figure 1 from Andrew Whiten, “Blind Alleys and Fruitful Pathways in the Comparative Study of Cultural Cognition,” Physics of Life Reviews (Vol. 43, 2022), 211-238; and Andrew Whiten, Jane Goodall, et al., “Charting Cultural Variation in Chimpanzees,” Behaviour (Vol. 138, No. 11/12, 2001), 1481-1516. Handclasp grooming is a charming arbitrary (i.e. having no particular adaptiveness) cultural behavior seen only among some chimpanzee populations where two mutually grooming chimpanzees hold opposite arms over their heads and hold hands, giving the other grooming access to their armpits. The rain dance is a behavior of male chimpanzees in some communities during rainstorms, when they put on an exaggerated display of seemingly deliberate physical movements, slapping the ground and drumming their feet while shouting and shaking vegetation. When multiple males do the rain dance together, they take turns. The rain dance varies considerably even among the different chimpanzee communities that do it: for the Taï chimpanzees, for example, it is a completely silent performance.
- I have little stake in this particular semantic debate. I do think, however, that identifying multiple clustered traditions is more a means of identifying discrete cultures, and is not as clearly getting to the heart of what culture as such is.
- As an example of the latter, see Bennett G. Galef and Kevin N. Laland, “Social Learning in Animals: Empirical Studies and Theoretical Models,” Bioscience (Vol. 55, No. 6, 2005), 489–99. The countercase in favor of a more expansive definition of culture is simply that the things we recognize as cultural in ourselves cannot actually be fully explained through only these processes of social learning. Kevin Laland (seemingly walking back his position from 2005), Jeremy R. Kendal, and Rachel L. Kendal write in “Animal Culture: Problems and Solutions” (chapter 8 from The Question of Animal Culture [2009], 180): “Nobody knows how much human social learning is actually dependent on the narrowly defined forms of imitation (reproducing through observation the specific motor patterns of another individual) or teaching (costly modification of behavior specifically designed to instruct a tutee). The fact that humans are capable of such narrow imitation and teaching is no reason to regard these as the sole or even principal processes of information transmission that underlie human culture.” If the matter of animals having culture were left off the table, I think it is clear that most everyone would recognize different human behaviors that are passed along by other, more basic mechanisms of social learning as cultural in nature.
- William McGrew, “Ten Dispatches from the Chimpanzee Culture Wars,” in The Question of Animal Culture (2009), 55.
- Or alternatively, “cultural evolution” or “cumulative cultural evolution.”
- I would like to note that this definition of cumulative culture as behaviors that no individual could possibly invent on their own has an important limitation. It muddles together the narrower means by which we can confidently identify cumulative culture with the more expansive process that we seek to identify, of successive innovative adaptations of learned behaviors. An excellent illustrative example of this is the Japanese macaques’ adoption of wheat-washing behavior after having learned to wash their sweet potatoes. Washing the wheat is itself a simple behavior, and it is perfectly plausible that an individual macaque could innovate this behavior without prompting from others, the same as Imo did with sweet potatoes. But it seems far more likely that this behavior was a second-order adaptation of what was learned from Imo rather than a standalone innovation: in other words, an example of cumulative culture.
- Michael Tomasello, The Cultural Origins of Human Cognition (Cambridge, MA, Harvard University Press, 1999).
- Peter J. Richerson and Robert Boyd, “Why Culture is Common, but Cultural Evolution is Rare,” Proceedings of the British Academy (Vol. 88, 1996), 82. Orangutan researcher Carel van Shaik writes, “Most…[experimentally demonstrated] social learning relies on very simple mechanisms, such as local enhancement or social facilitation, perfect for the transmission of information but not for that of true innovations, that is, novel behavioral variants that arise rather rarely because of some process of exploration and invention” (“Geographic Variation in the Behavior of Wild Great Apes: Is It Really Cultural?,” in The Question of Animal Culture [2009], 71.
- Richerson and Boyd, “Why Culture is Common, but Cultural Evolution is Rare,” (1996): “Most culture in non-human animals involves behaviours that individuals can, and do, learn on their own. There are only a few well documented cases in which cultural change accumulates over many generations leading to the evolution of behaviours that no individual could invent—the only well documented examples are song dialects in birds, perhaps some behaviours in chimpanzees, and of course many aspects of human behaviour.”
- In the south Pacific, for instance, this influence over what song is “in” flows from west to east, with musical innovations by the humpbacks off the east Australian coast coming to blanket the entire Pacific (by which point they have moved on to their own new song). See Ellen C. Garland et al., “Dynamic Horizontal Cultural Transmission of Humpback Whale Song at the Ocean Basin Scale,” Current Biology (Vol. 21, No. 8, 2011), 687-691.
- Described by Ellen Garland in her lecture at the SETI Institute, “Whales, Their Song, Their Culture: Another Intelligence on Earth,” available on YouTube: https://www.youtube.com/watch?v=3QO_UWxglm0. See also Michael J. Noad, et al., “Cultural Revolution in Whale Songs,” Nature (Vol. 408, No. 537, 2000).
- Bubble net feeding was first systematically documented by the doctoral research of Fred Sharpe in British Columbia. He both observed humpbacks at work and experimentally tested different aspects of their technique. Herring, he found, would flee away from the humpback’s hunting cry and clump tightly together when surrounded by bubbles, while waving flippers (tested with marionette-style puppet ones) would keep any from escaping. His dissertation, “Social Foraging of the Southeast Alaskan Humpback Whale, Megaptera novaeangliae,” is available here: https://www.alaskahumpbacks.org/research/Sharpe_dissertation.pdf. Earlier accounts of a form of bubble net feeding (solo, as is the practice for humpbacks in the Atlantic) go back to at least the beginning of the twentieth century. In 1905, Norwegian whaler Morten Andreas Ingebrigtsen described a humpback in the Norwegian Arctic “a short distance below the surface of the water, swimming in a ring while at the same time it blew off. The air rose to the surface like a thick wall of air bubbles and these formed the ‘net.’ The ‘krill’ saw this wall of air bubbles, were frightened into the centre.” The whale then “came up in the centre to fill its open mouth with ‘krill’ and water, after which it lay on its side, closed its mouth, and the catch was completed.” Quoted by Whitehead and Rendell in The Cultural Lives of Whales and Dolphins (2015), 94.
- I am skirting over the sheer complexity of this task for reasons of space. Creating this rising spiral relies on incredibly precise technique. Sharpe found that humpbacks were producing two different kinds of bubbles that work together to create a barrier: large rounded ones (100-500 mL) that structure the top of the bubble net, which he calls “spherical cap leaders,” and streams of smaller bubbles (2-30 mL) trapped beneath them, which he calls “effervescence.” These bubbles must be perfectly timed with the depth from which they are released (often around fifty feet beneath the surface), the speed of the fish they are surrounding, and the number of other whales participating in the bubble netting crew. Imprecision with these variables can result in a patchy bubble net, from which fish can escape. It is not fully understood how exactly humpbacks are able to consciously form different kinds of bubbles, but it must entail some kind of fine motor control with the blowhole.
- Leigh Calvez, The Breath of a Whale (Seattle, Sasquatch Books, 2019), 31-34.
- ibid.
- Fred Sharpe, quoted in Elin Kelsey, Watching Giants: The Secret Lives of Whales (Berkeley, University of California Press, 2008), 59. Sharpe argues that bubble net feeding is a form of tool use, manipulating air and water in precise, goal-oriented ways beyond the body. He states: “Bubble net feeding is a form of tool using behavior. The humpbacks are perhaps unique, with humans, in that it’s a form of communal tool use. One individual will employ a net for the entire group” (ibid 57). Definitions of “tools” and “tool use” are of course fraught and complex, often including behaviors not intuitively imagined as tool use in more vernacular understandings as well as not including behaviors that almost everyone would agree constitute tool use. Benjamin Beck’s 1980 definition (“the external employment of an unattached environmental object to alter more efficiently the form, position, or condition of another object, another organism, or the user itself when the user holds or carries the tool during or just prior to use and is responsible for the proper and effective orientation of the tool”) has been the most influential, but it has problems. “Object” does not have a wholly unambiguous meaning (especially when we are talking about humpback whales making use of air and water), and it inadequately accounts for tool use that is for information gathering about rather than manipulation of one’s surroundings, as with gorillas testing water depth with a stick. See Robert St Amant and Thomas E. Horton, “Revisiting the Definition of Animal Tool Use,” Animal Behaviour (Vol. 75, No. 4, 2008), 1199-1208. See also Doug Perrine, “For Humpbacks, Bubbles Can Be Tools,” Hakai Magazine (December 20, 2022). https://hakaimagazine.com/videos-visuals/for-humpbacks-bubbles-can-be-tools/.
- Whitehead and Rendell, The Cultural Lives of Whales and Dolphins (2015), 94.
- Further research also found that the prevalence of lobtail feeding in a given season tracked the availability of sand lance, leading to the conclusion that lobtail feeding is a specialized method for that particular prey. See Whitehead and Rendell, The Cultural Lives of Whales and Dolphins (2015), 95, and J. Allen et al., “Network-based Diffusion Analysis Reveals Cultural Transmission of Lobtail Feeding in Humpback Whales,” Science (Vol. 340, 2013), 485-488.
- Tim Vernimmen, “Cultural Transmission Makes Animals Flexible, but Vulnerable,” Knowable Magazine (July 29, 2022). https://knowablemagazine.org/content/article/living-world/2022/cultural-transmission-makes-animals-flexible-vulnerable.
- An early version of this appears in the work of primatologist William McGrew, where he refers to it as the “ethnographic method”—though “comparative ethnographic method” might better describe his research process, which entailed systematic documentation and comparison of suites of chimpanzee behaviors between different groups. In their preface to The Biology of Traditions: Models and Evidence, Dorothy Fragaszy and Susan Perry refer to it as the “group contrast method” (Cambridge, Cambridge University Press, 2003). Elsewhere it is also referred to as the “method of exclusion.”
- Andrew Whiten et al., “Cultures in Chimpanzees,” Nature (Vol. 399, 1999), 682-685.
- Such false negatives could result from multiple possible conditions. A given behavior may be socially transmitted vertically (from parents to offspring), and thereby circulate through a subpopulation in precisely the same way as given genetic markers. With such correlation, we might falsely attribute the behavioral difference to genetics (or at least not attribute it to culture, on the grounds that it is “plausibly genetic”). Or a socially learned trait may have spread so successfully that it becomes universal in a given well-integrated population. Utilizing the elimination method, such a trait would be indistinguishable from a genetic characteristic of the species as a whole. Or a cultural behavior has gene-culture coevolutionary effects, such that it shifts selective pressures and drives genetic changes. This too would be mistaken for a genetic trait. Or most commonly, a cultural behavior may be an adaptive response to a particular environment, creating a correlation between expression of that behavior and local ecological factors, which would lead us to mistake it as resulting from individual (non-social) learning under conditions of environmental variation. Kevin Laland, Jeremy Kendal, and Rachel Kendal summarize these problems, stating, “…we suspect that were the ethnographic approach [elimination method] to be rigorously applied, it would reject most genuine cases of culture. Correlations between behavioral and ecological variables are to be expected because culture is a source of adaptive behavior, which enables animals to learn about and exploit environmental resources. Similarly, cultural and genetic covariance is also anticipated because animal learning is influenced by evolved predispositions and aptitudes.” From Kevin N. Laland, Jeremy R. Kendal, and Rachel L. Kendal, “Animal Culture: Problems and Solutions” in The Question of Animal Culture (2009), 185-186.
- Whitehead and Rendell, The Cultural Lives of Whales and Dolphins (2015), 43.
- In “Animal Culture: Problems and Solutions” (in The Question of Animal Culture [2009], 174-197), Kevin Laland, Jeremy Kendal, and Rachel Kendal put forward the following: bootstrapping sampling distributions for the asocial learning of novel tasks (essentially using statistical methods to see if animals in a given population are collectively biased towards a given strategy or behavior despite viable alternatives); signatures of social learning found by analyzing diffusion curves of the prevalence and spread of particular behaviors to see whether they match the statistical “signatures” of cultural transmission (rates, pathways, and patterns of spread that are diagnostic of social learning); fitting models to diffusion data by developing models of how social learned behaviors spread by way of different transmission mechanisms, and seeing which actually match data on their prevalence and spread; fitting models to inheritance data, using regression models to represent what mix of genetic, environmental, and cultural influences there are on behavior, and whether that cultural transmission is vertical, oblique, or horizontal/conformist; partial regression permutation tests (a method developed by Hal Whitehead, to test how much interindividual variation in behavior is caused by “social similarity,” i.e. degree of social relationship, over and above their ecological and/or genetic similarity); and phylogenetic analyses, whereby behavioral differences between and within subspecies are compared to clarify how significant a factor genetic heritability might or might not be. In another chapter of the same book (“Intrapopulation Variation in Bottlenose Dolphins,” 152-173), Brooke Sergeant and Janet Mann suggest their own list of statistical and research methods with a number of sources exemplifying them: the use of transmission chains and diffusion curves as a social learning diagnostic method (citing R.L. Day, J.R. Kendal, and Kevin N. Laland, “Validating Cultural Transmission in Cetaceans: Reply to Rendell and Whitehead,” Behavioral and Brain Sciences, [Vol.24, 2001], 330-331; and Kevin N. Laland and J.R. Kendal, “What the Models Say about Animal Social Learning,” in The Biology of Traditions: Models and Evidence, eds. Dorothy M. Fragaszy and Susan Perry [Chicago, University of Chicago Press, 2003], 33-55), correlations between association and behavioral similarity (citing S. Perry et al, “Traditions in wild white-faced capuchin monkeys,” in The Biology of Traditions: Models and Evidence, 391-425), field experiments (citing T. Humle and T. Matsuzawa, “Ant-dipping among the chimpanzees of Bossou, Guinea, and some comparisons with other sites,” American Journal of Primatology [Vol. 58, 2002], 133-148; S.M. Reader, J.R. Kendal, and K.N. Laland, “Social learning of foraging sites and escape routes in wild Trinidadian guppies,” Animal Behaviour, (Vol. 66, 2003), 729-739; and B.G. Galef, Jr., “Approaches to the study of traditional behaviors of free-living animals,” Learning and Behavior, [Vol. 32, 2004] 52-61.), Comparison of field data to experimental work on captive populations of theoretical models (J. Terkel, “Cultural transmission of feeding behavior in the black rat [Rattus rattus],” in Social Learning in Animals: The Roots of Culture, eds. C.M. Heyes and B.G. Galef Jr. [San Diego, Academic Press, 1996], 17-48; Kevin N. Laland and J.R. Kendal, “What the models say about animal social learning”; G. Dewar, “The cue reliability approach to social transmission: Designing tests for adaptive traditions,” in The Biology of Traditions: Models and Evidence; and G. Dewar, “Social and asocial cues about new food: Cue reliability influences intake in rats,” Learning and Behavior, [Vol. 32, 2004], 82-89), and use of multifactorial models that measure the contributions of multiple factors to behavioral development and avoid the concept of exclusion (B.L. Sargeant, “Foraging development and individual specialization in wild bottlenose dolphins [Tursiops sp.],” PhD thesis, Georgetown University [2005].)
- Sabine Coussi-Korbel and Dorothy M. Fragaszy, “On the Relation Between Social Dynamics and Social Learning,” Animal Behaviour (Vol. 50, No. 6, 1995), 1441-1453; and Carel P. van Shaik, Robert O. Deaner, and Michelle Y. Merrill, “The Conditions for Tool Use in Primates,” Journal of Human Evolution (Vol. 36, No. 6, 1999), 719-741.
- Julio Mercader et al., “4,300-Year-old Chimpanzee Sites and the Origins of Percussive Stone Technology,” PNAS (Vol. 104, No. 9, 2007), 3043-3048. This is not necessarily a death knell for cumulative culture in chimpanzees. Humans, the poster children for cumulative cultural excellence, have also lived through thousands of years with little or no technological change that is archaeologically discernible.
- This line of argument is largely spearheaded by the comparative psychologist Michael Tomasello. To clarify further for any unfamiliar with these terms, the wings of a bat and the wings of a bird are analogous structures. They evolved independently and are modifications on different underlying anatomical structures (the fingers for a bat and the arms for a bird). A seal’s flippers and a human’s hand, however, are homologous, despite having very different functions.
- Bookchin, Re-enchanting Humanity (1995), 25.
- It is worth noting also that Bookchin’s descriptions of chimpanzees’ lack of altruism and social care fall outside current consensus in the scientific literature. See, for instance, Edwin J.C. van Leeuwen et al., “Chimpanzees Behave Pro-Socially in a Group Specific Manner,” Science Advances (Vol. 7, No. 9, 2021); and Ed Yong, “Altruistic Chimpanzees Clearly Help Each Other Out,” National Geographic (May 23, 2009), https://www.nationalgeographic.com/science/article/altruistic-chimpanzees-clearly-help-each-other-out.
- Lucy Anne Bates, Joyce H. Poole, and Richard W. Byrne, “Elephant Cognition”, Current Biology (Vol. 18, No. 13, 2008), 545-546.
- Phyllis C. Lee, “Allomothering Among African Elephants,” Animal Behaviour (Vol. 35, No. 1, 1987), 278–291.
- As do, notably, humans, and not only in our post-agricultural age of high population densities. (See, for extended discussions of seasonal convergence and dispersal of hunter-gatherer populations, David Graeber and David Wengrow, The Dawn of Everything: A New History of Humanity [London, Farrar, Straus, and Giroux, 2021].) Other species with fission-fusion societies include wolves, some apes, dolphins, and other cetaceans.
- Or at least most typically of related adult females. Females are known to occasionally be adopted into new families other than the one they were born into.
- There are also documented cases of families headed by something like co-chairs: two elder females, usually sisters or cousins, who lead jointly, each taking on different aspects of leadership and/or making key decisions jointly.
- Harvey Croze and W. Keith Lindsay, “Amboseli Ecosystem Context: Past and Present,” chapter 2 from The Amboseli Elephants: Long Term Study of a Long-Lived Animal, eds. Cynthia J. Moss, Harvey Croze, and Phyllis C. Lee (Chicago, University of Chicago Press, 2011), 38.
- G. Wittemyer, I. Douglas-Hamilton, and W.M. Getz, “The socioecology of elephants: analysis of the processes creating multitiered social structures,” Animal Behavior (Vol. 69, No. 6, 2005), 1366.
- Veteran elephant researchers Harvey Croze and Cynthia Moss describe them as “not based on kinship but apparently on an elusive quality that it is tempting to term ‘friendship.’” Harvey Croze and Cynthia Moss, “Patterns of Occupancy in Time and Space,” from The Amboseli Elephants(2011), 101.
- Cynthia J. Moss, “Social Circles,” Wildlife News (Vol. 16, 1981),2-7. “Clan” is unfortunately a confusing term in the context of this essay, for all three species I am doing a deep dive into have large-scale social organizations termed “clans,” which means something markedly different in each case.
- Wittemyer, Douglas-Hamilton, and Getz, “The Socioecology of Elephants” (2005), 1366.
- Richard W. Byrne and Lucy A. Bates, “Elephant Cognition in Primate Perspective,” Comparative Cognition and Behavior Reviews (Vol. 4, 2009), 65-79. https://comparative-cognition-and-behavior-reviews.org/wp/wp-content/uploads/2013/10/vol_4_byrne.pdf. In a similar article (“Elephant Cognition,” Current Biology [Vol. 18, No. 13, 2008] R544-546), these two authors are joined by Joyce H. Poole and write, “As a non-specialist browser, too large to be much threatened by predators, an elephant’s biggest cognitive challenge is most likely to be social. All species of elephants form large social networks, with hierarchical, multi-level organisation, implying that elephants can deal with a degree of social complexity.” For a social ecology audience, it is worth noting that “hierarchical” is not used in this literature in the anthropological or political sense of a command and control relationship, but rather a multiscalar system of organization: mother-calf relationships are nested within families, which are nested in bond groups, nested within clans, which are in turn nested within sub-populations and populations.
- Karen McComb, et al., “Unusually Extensive Networks of Vocal Recognition in African Elephants,” Animal Behaviour (Vol. 59, No. 6, 2000), 1103-1109.
- See Joyce H. Poole, “Behavioral Contexts of Elephant Acoustic Communication,” chapter 9 in The Amboseli Elephants (2011), 125-161.
- ibid 155-156. I will here quote Poole at length, for her descriptions, though conveyed in a level-headed and strictly scientific tone, are simply extraordinary. She writes, “Not uncommonly, vocal exchanges between related adult females may be heard that have the cadence of a conversation, rising and falling, as first one individual and then another contributes her voice. Other females may join the initiating individuals in a sequence of low-pitched, moderate intensity, relatively flat, long rumbles… The patterning of vocal exchange has such a cadence of conversation (in particular, what sounds like a higher pitch/lower pitch exchange) that I refer to this as a cadenced-rumble. These calls may overlap, although typically the degree of overlap is small. Adult females appear to use cadenced-rumbles to ‘lend their voice to’ a proposed plan of action, usually, it seems, regarding where to go and when to depart (e.g., related to ‘let’s-go’–rumbling)… Cadenced-rumbles are often heard following a series of ‘let’s-go’ calls and a series of cadenced-rumbles may contain calls that are difficult to distinguish from ‘let’s-go’-rumbles. This may be because there are ‘let’s-go‘-rumbles interspersed among the other calls or because they are one and the same call. Much additional research is needed to clarify the temporal pattern and usage of these calls, but based on the behavior of the elephants the cadenced-rumble appears to represent a complex level of consensus building between family members. It appears as if one individual proposes a course of action (using the ‘let’s-go‘-rumble, for example) and then a period of negotiation and consensus building follows, ending in either concordance or disagreement. Agreement is sometimes evident when individuals gather together rumbling with heads raised and touching or when individuals who were suggesting different directions of travel compromise on one direction. Alternatively, the elephants may continue to disagree and go separate ways.” (Certain parentheticals removed for clarity of reading out of context.)
- Joyce H. Poole, et al., “Elephants Are Capable of Vocal Learning,” Nature: Brief Communications (Vol. 434, 2005), 455-456. https://www.elephantvoices.org/phocadownload/Poole_et_al_2005_vocal_learning.pdf. One captive Asian elephant in South Korea even managed to utter a few recognizably Korean words. See Shannon Fischer, “Elephant ‘Speaks’ Like a Human—Uses Trunk to Shape Sound,” National Geographic (November 3, 2012). https://www.nationalgeographic.com/animals/article/121102-korean-speaking-elephant-talk-human-science-weird-animals.
- Croze and Moss, “Patterns of Occupancy in Time and Space” (2011), 125.
- Tom Reuter, Sirpa Nummela, and Simo Hemilä, “Elephant Hearing,” Journal of the Acoustical Society of America (Vol. 104, 1998), 1122-1123; Caitlin O’Connell, Lynette A. Hart, and Byron T. Arnason, “Comments on ‘Elephant Hearing,'” Journal of the Acoustical Society of America (Vol. 105, No. 3, 1998), 2051-2052.
- Carl Safina writes that “In a privately owned wildlife sanctuary in Zimbabwe lived some eighty well-known, very relaxed elephants who hung around a tourist lodge’s artificial water holes. Officials ninety miles away in Hwange National Park decided to reduce the park’s elephant densities by ‘culling’ hundreds of elephants (using helicopters to herd elephants to waiting marksmen, who were instructed to kill whole families). On the day the distant slaughter started, the relaxed tourist-lodge elephants abruptly vanished. Several days later, they were found bunched together in the corner of the sanctuary farthest from Hwange.” (In Beyond Words: What Animals Think and Feel [New York, Henry Holt and Company, 2015], 92.)
- Elephants have the largest olfactory lobe of any animal, and they vastly outstrip dogs and other expert smellers in the variety and density of their smell receptors. For sources on their ability to identify known individuals by urine scent, see Hannah Mumby, Elephants: Birth, Life, and Death in the World of the Giants (New York, HarperCollins, 2020), 34; and Lucy Anne Bates et al., “African Elephants Have Expectations About the Locations of Out-of-Sight Family Members,” Biology Letters (Vol. 4, No. 1, February 2008). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2412944/.
- “Chemical communication,” Elephant Voices. https://www.elephantvoices.org/elephant-communication/chemical-communication.html.
- Depicted here: Do Elephants Have Culture? – Londolozi Blog.
- Extreme, at least, by mammalian standards. Many fish, birds, and a whole host of various invertebrates make any differences between the sexes of mammals seem imperceptible by comparison.
- The word enters English from the Persian word for “drunk.”
- Rob Slotow et al., “Older Bull Elephants Control Young Males,” Nature (Vol. 408, 2000), 425-426; Carl Safina, Beyond Words, 46.
- Lucy Bates, et al., “Why Do African Elephants (Loxodonta africana) Simulate Oestrus? An Analysis of Longitudinal Data,” PLoS ONE (Vol. 5, No. 4, 2010).
- Levison Wood, “A Curious Mind,” Chapter 6 from The Last Giants: The Rise and Fall of the African Elephant (New York, Grove Press Black Cat, 2020).
- “Elephants Learn From Others,” Elephant Voices, https://www.elephantvoices.org/elephant-sense-a-sociality-4/elephants-learn-from-others.html.
- ibid
- Croze and Moss, “Patterns of Occupancy in Time and Space” (2011), 123
- Joyce H. Poole and Petter Granli, “Signals, Gestures, and Behaviors of African Elephants,” chapter 8 in Amboseli Elephants (2011), 113; and Joyce H. Poole, “Behavioral Context of Elephant Acoustic Communication,” chapter 9 in Amboseli Elephants (2011), 151.
- Safina, Becoming Wild (2020), 23.
- Charles Foley, Nathalie Pettorelli, and Lara Foley, “Severe Drought and Calf Survival in Elephants,” Biology Letters (Vol. 4, No. 5, 2008), 541-544. This paper studied survival during the 1993 drought in Tsavo National Park in Tanzania, which determined that calves were significantly likelier to survive if their family had females who had lived through the 1958-61 drought.
- Stephen Blake, et al., “The Last Sahelian Elephants: Ranging Behaviour, Population Status and Recent History of the Desert Elephants of Mali,” Save the Elephants (August 2003); and P.J. Viljoen, “Spatial Distribution and Movements of Elephants (Loxodonta africana) in the Northern Namib Desert Region of Kaokoveld, South West Africa/Namibia,” Journal of Zoology (Vol. 219, 1989), 1-19.
- Among bottlenose dolphins, these include strand feeding (a risky tactic of deliberate self-beaching to catch fish, witnessed on the shores of Kiawah Island, shown here), kerplunking (a method of startling fish with a concussive splash of the tail, shown here, beginning 8:20), mud-net fishing (where a dolphin beats the sea floor with their tail while circling a shoal of fish in shallow water, trapping them in a ring of mud, shown here), hydroplaning (literally gliding over the sand by hydroplaning to catch fish at the shoreline, shown here), herding (a collective hunting strategy of frightening fish into a tight swarm at the surface, shown here), bottom-grubbing (hunting vertically, tail up and nose down, at the ocean floor, shown here, beginning at 3:15), sponging (carrying a sponge over their rostrum to protect themselves from rough corals while foraging, shown here), stingray following (taking advantage of the stingray’s ability to find prey hiding in seagrass using electroreceptors, revealing and flushing out some of dolphins’ favorite food, shown here), shelling or conch carrying (chasing a fish into a conch shell, which is then carried to the surface and lifted out of the water, draining the water out and pouring the hiding prey straight into one’s mouth, shown here, discussed further in S.J. Allen, L. Bejder, and M. Krützen, “Why do Indo-Pacific bottlenose dolphins [Tursiops sp.] carry conch shells [Turbinella sp.] in Shark Bay, Western Australia?,” Marine Mammal Science, [Vol. 27, No. 2, 2011], 449-454), and perhaps most amazingly, cuttlefish processing (a multistage “recipe” of rendering a cuttlefish edible, requiring the deliberate release of its defensive ink and the removal of the cuttlebone, diagrammed here, from Julian Finn, Tom Tregenza, and Mark Norman, “Preparing the Perfect Cuttlefish Meal: Complex Prey Handling by Dolphins,” PLoS ONE [Vol. 4, No. 1, 2009], e4217, where there is further discussion).
- The Brazilian dolphins signal to the fisherman where to cast their nets (shown here); in Mauritania, it is the fisherman who summon the dolphins to herd the fish into their nets (Carl Safina, Beyond Words [2015], 260). Aboriginal peoples of eastern Australia have achieved these cooperative relationships with both bottlenose dolphins and killer whales. Aboriginal Australians have a close cultural relationship with killer whales in particular. Oral histories seemingly dating to the last glacial maximum when sea levels were considerably lower recount hunting grounds that have since disappeared under the advancing waves, and some Aboriginal peoples understand killer whales as their ancestors, returning to hunt in their old territories. See David T. Neil, “Cooperative Fishing Interactions Between Aboriginal Australians and Dolphins in Eastern Australia,” Anthrozoös (Vol. 15, No. 1, 2002), 3-18. In each of these three cases the catch is shared between both species.
- See Brooke L. Sergeant and Janet Mann, “Intrapopulation Variation in Bottlenose Dolphins,” in: The Question of Animal Culture, (Cambridge, Massachusetts, Harvard University Press, 2009), p. 152-173; J. Mann et al., “Female reproductive success in bottlenose dolphins (Tursiops sp.): Life history, habitat, provisioning, and group-size effects,” Behavioral Ecology (Vol. 11, 2000), 210-219; J. Mann and B.L. Sargeant, “Like mother, like calf: The ontogeny of foraging traditions in wild Indian Ocean bottlenose dolphins (Tursiops sp.), in: The Biology of Traditions: Models and Evidence (2003); Connor et al, “The bottlenose dolphin: Social relationships in a fission-fusion society,” in Cetacean Societies: Field Studies of Dolphins and Whales (2000); and Gazda et al., “A division of labour with role specialization in group-hunting bottlenose dolphins (Tursiops truncatus) off Cedar Key, Florida” Proceedings of the Royal Society of London, Series B (Vol. 272, 2005), 135-140.
- Despite the name, killer whales are the world’s largest species of dolphin. They are also known as “orcas,” from their Latin name Orcinus orca. “Orca” may sound to English-speaking ears as a less derogatory label, but it is little better: Orcinus orca means “demon of the underworld.”
- Safina, Beyond Words (2015), 332.
- There is less conclusive evidence for menopause (or something approaching menopause) in other matrilineal cetaceans—including sperm whales. See Peter B. Best, P.A.S. Canham, and N. Macleod, “Patterns of reproduction in sperm whales, Physeter macrocephalus,” Reports of the International Whaling Commission (Special Issue) (Vol. 6, 1984), 51–79. Whitehead and Rendell summarize (The Cultural Lives of Dolphins and Whales, 2015): “in the data set compiled by Peter Best and his colleagues from whaling off South Africa, the oldest pregnant sperm whale was forty-one years old while there were twenty-two other females aged between forty-two and sixty-one… Interestingly, six of the older nonreproductive female sperm whales were lactating, so they are contributing physiologically to new generations without giving birth themselves.”
- Stuart Nattrass, et al., “Postreproductive killer whale grandmothers improve the survival of their grandoffspring,” PNAS (Vol. 116, No. 52, 2019), 26669-26673. The importance of older females extends even beyond juveniles. At least in resident killer whales (discussed more at length later on), mothers are essential to the continued survival of their adult sons. The mortality rate of an adult male under the age of thirty whose mother has died is three times as high as one of the same age with a living mother. If they are over thirty, their mortality spikes eightfold after the loss of their mother. (Safina, Beyond Words [2015], 330).
- See Natalie Angier, “Theorists See Evolutionary Advantages in Menopause,” The New York Times (16 September 1997); Jared Diamond, “Why Women Change,” Discover (30 June 1996); and Kristen Hawkes, James O’Connell, and Nicholas Blurton Jones, “Hadza Women’s Time Allocation, Offspring Provisioning, and the Evolution of Long Postmenopausal Life Spans,” Current Anthropology (Vol. 38, No. 4, 1997), 551-557.
- For a more detailed discussion elaborating (among other things) why this has not been the case for elephants, where elder females are similarly essential for their accumulated knowledge and allomothering responsibilities, see Ed Yong, “Why Killer Whales (and Humans) Go Through Menopause,” The Atlantic (12 January 2017). https://www.theatlantic.com/science/archive/2017/01/why-do-killer-whales-go-through-menopause/512783/.
- In the scientific literature, two individuals are recognized as belonging to the same pod if they are together during at least 50% of observations.
- V.B. Deecke, J.K.B. Ford, and P. Spong, “Dialect Change in Resident Killer Whales: Implications for Learning and Cultural Transmission,” Animal Behaviour (Vol. 60, 2000), 629-638; and Andrew D. Foote et al., “Killer Whales Are Capable of Vocal Learning,” Biology Letters (Vol. 2, No. 4, 2006), 509-512.
- You can listen to these various vocalizations and hear how they differ between pods here.
- H. Yurk, et al., “Cultural transmission within maternal lineages: Vocal clans in resident killer whales in southern Alaska,” Animal Behaviour (Vol. 63, 2002), 1103–1119.
- The thirty-four pods of the northern residents are divided into three clans: A Clan, G Clan, and R Clan. The southern residents are much fewer in number (and in considerably more danger of inbreeding, population collapse, and extinction) and have only three pods, all of which belong to the J Clan. There are two known clans among the Alaska residents, AB and AD. The Alaska residents are possibly a little bit more flexible with the fish they eat. They are sometimes known to eat halibut and cod, though remain primarily specialized for salmon.
- Whitehead and Rendell, Cultural Lives of Whales and Dolphins (2015), 144.
- Hal Whitehead, et al., “Culture and Conservation of Non-Humans with Reference to Whales and Dolphins: Review and New Directions,” Biological Conservation (Vol. 120, 2004), 431–41.
- John K.B. Ford, Graeme M. Ellis, and Kenneth C. Balcomb, Killer Whales: The Natural History and Genealogy of Orcinus Orca in British Columbia and Washington State (Vancouver, University of British Columbia Press, 2000).
- That, at least, is the evolutionary explanation. Attraction to those with a different accent or sexual taboos against mating with those of one’s same vocal dialect may very well be experienced by actual killer whales differently than this cold genetic calculus (i.e. as a source of sex appeal).
- They are also known as Bigg’s Killer whales, named in honor of Michael Bigg, the pioneer of modern killer whale research.
- See John K. B. Ford and Graeme M. Ellis, Transients: Mammal-Hunting Killer Whales (Vancouver, University of British Columbia Press, 1999), 83.
- Phillip A. Morin, et al., “Geographic and temporal dynamics of a global radiation and diversification in the killer whale,” Molecular Ecology (Vol. 24, No. 15, 2015), 3964–3979. Mitochondrial DNA is only inherited from the mother, so it can provide clear records of matrilineal ancestry. It cannot tell us about the reproductive behavior of males (i.e. whether or not they continued to mate across communities), but it shows the social split between matrilineal family groups. Other killer whale ecotypes around the world are further diverged, having no common female ancestors more recent than 125,000 years ago, and possibly as long as 227,000 years ago.
- Robin W. Baird and Hal Whitehead, “Social organization of mammal-eating killer whales: group stability and dispersal patterns,” Canadian Journal of Zoology (Vol. 78, No. 12, 2000), 2096–2105; and Robin W. Baird and Lawrence M. Dill, “Ecological and Social Determinants of Group Size in Transient Killer Whales,” Behavioral Biology (Vol. 7, No. 4, 1996), 408-416.
- Volker B. Deecke, John K.B. Ford, and Peter J.B. Slater, “The Vocal Behaviour of Mammal-Eating Killer Whales: Communicating with Costly Calls,” Animal Behaviour (Vol. 69, No. 2, 2005), 394-405.
- Sara B. Tavares, Filipa I.P. Samarra, and Patrick J.O. Miller, “A multilevel society of herring-eating killer whales indicates adaptation to prey characteristics,” Behavioral Ecology (Vol. 28, No. 2, 2017), 500-514.
- Craig Matkin and John Durban, “Killer Whales in Alaskan Waters,” Whalewatcher (Vol. 40, No. 1, 2011), 27-28. https://swfsc-publications.fisheries.noaa.gov/publications/CR/2011/2011Matkin.pdf.
- Craig Matkin and John Durban, “Killer Whales in Alaskan Waters,” Whalewatcher (Vol. 40, No. 1, 2011), 24-29. https://swfsc-publications.fisheries.noaa.gov/publications/CR/2011/2011Matkin.pdf. The GOA transients have, however, been observed socially engaging with the West Coast transients (those that travel through the same waters as the northern and southern residents in and around the Salish Sea) exactly once. Populations of transients may be thought of as roughly analogous to the communities of residents, but these cultural barriers should not be thought to map precisely onto one another.
- Robin W. Baird and Lawrence M. Dill, “Ecological and Social Determinants of Group Size in Transient Killer Whales,” Behavioral Biology (Vol. 7, No. 4, 1996), 408-416.
- Craig Matkin and John Durban, “Killer Whales in Alaskan Waters,” Whalewatcher (Vol. 40, No. 1, 2011), 28. https://swfsc-publications.fisheries.noaa.gov/publications/CR/2011/2011Matkin.pdf.
- Margaret M. Krahn, et al., “Use of chemical tracers in assessing the diet and foraging regions of eastern North Pacific killer whales,” Marine Environmental Research (Vol. 63, 2007) 91–114.
- This is especially significant in light of the estimate that there are only around 300 Pacific offshore killer whales in total. See Craig Matkin and John Durban, “Killer Whales in Alaskan Waters” (2011); and John K.B. Ford et al., “Offshore Killer Whales in Canadian Pacific Waters: Distribution, Seasonality, Foraging Ecology, Population Status and Potential for Recovery,” Department of Fisheries and Oceans Canadian Sciences Advisory Secretariat (2014).
- See Figure 17 of John K.B. Ford et al. 2014 (page 50 here).
- Icelandic Type 1 killer whales (the predominantly herring-eaters) are set starkly apart from north Pacific ecotypes in that there is in-group variance in prey preference. Some eat herring year-round, while others seasonally make the switch to hunting seals, but all of them are still treated as “us.” See Filipa I.P. Samarra et al., “Intra-population Variation in Isotropic Niche in Herring-eating Killer Whales off Iceland,” Marine Ecology Progress Series (Vol. 564, 2017), 199-210; and Filipa I.P. Samarra et al., “Prey of Killer Whales (Orcinus orca) in Iceland,” PLoS One (Vol. 13, No. 12, 2018). From Samarra et al. 2017: “Unlike sympatric killer whale populations of different ecotypes described in other areas that are ecologically, socially and genetically isolated, in Iceland ecological specialisation does not appear to occur at the population level. Instead, groups within the population appear to share part of their ecological niche at least seasonally. This shared niche, together with temporary social associations, may mean that there is no genetic divergence between the different groups described here and, thus, that different movement patterns and foraging traditions may be maintained without genetic divergence.”
- See Sara B. Tavares, Filipa I.P. Samarra, and Patrick J.O. Miller, “A multilevel society of herring-eating killer whales indicates adaptation to prey characteristics,” Behavioral Ecology (Vol. 28, No. 2, 2017), 500-514 for a statistical analysis of the social structure of the Icelandic killer whales.
- For a discussion on why this remains an open question, see Andrew D. Foote, “Are ‘Type 2’ Killer Whales Long in the Tooth?: A Critical Reflection on the Discrete Categorization of Northeast Atlantic Killer Whales,” Marine Mammal Science (Vol. 39, 2023), 345-350.
- Most commonly, scientists describe these as distinct ecotypes. However, we know so little about them that other scientists caution against this as a premature application of categories developed from killer whale populations in the northern hemisphere, which may distort our analysis of the plausibly quite different cultural makeup of the southern hemisphere. See P.J.N. de Bruyn, Cheryl A. Tosh, and Aleks Terauds, “Killer Whale Ecotypes: Is There a Global Model?,” Biological Reviews (Vol. 88, No. 1, 2013), 62-80. These authors point to observations of photo-identified individuals exhibiting morphological characteristics and prey types associated with both Type A and Type Bs, for instance. They argue that Antarctic killer whales may be more generalist hunters than perceived by other researchers approaching their study with a North Pacific-informed ecotype model in mind.
- Robert L. Pitman and Paul Ensor, “Three forms of killer whales (Orcinus orca) in Antarctic waters,” Journal of Cetacean Research and Management (Vol. 5, No. 2, 2003), 131-139.[/efn_note} Type B1 uses a cooperative hunting strategy called “wave crashing.” One individual swims up to a floe of pack ice and peaks their head above the water to find a Weddell seal. (Crabeater seals, who account for nearly 85% of the region’s seal population, are ignored.)144Robert L. Pitman and John W. Durban “Cooperative hunting behavior, prey selectivity and prey handling by pack ice killer whales (Orcinus orca), type B, in Antarctic Peninsula waters,” Marine Mammal Science (Vol. 28, No. 1, 2012), 16–36.
- Observations suggest this technique requires training to master. Swedish marine biologist Olle Carllson recounts witnessing a group of Type B1 killer whales around the Antarctic Peninsula knock a single seal off its ice floe five times in a row, each time swimming away and allowing the seal to clamber back up into relative safety. This was done in the presence of two calves, and likely an exercise in teaching. Personal communication cited in Robert L. Pitman and Paul Ensor, “Three forms of killer whales (Orcinus orca) in Antarctic waters,” Journal of Cetacean Research and Management (Vol. 5, No. 2, 2003), 131-139.
- In extremely cold water, killer whales limit their heat loss by maintaining a severe temperature gradient between their skin layer and deeper layers of blubber. This means, however, that blood flow to the skin is minimal. Warmer waters would allow them to send blood back to their skin for healing without facing a severe heat loss energy cost. These migrations are brief, with the individuals in question traveling directly and at high speeds. One geotagged killer whale made the 9400 km round trip in just 42 days. The physiological maintenance hypothesis would explain why Antarctic killer whales often appear yellowish, their skin infested with diatoms, but the same individuals later appear “clean” and white. John W. Durban and Robert L. Pitman, “Antarctic Killer Whales Make Rapid, Round-trip Movements to Subtropical Waters: Evidence for Physiological Maintenance Migrations?,” Biology Letters (Vol. 8, 2012), 274-277.
- Robin W. Baird, et al., “Killer Whales in Hawaiian Waters: Information on Population Identity and Feeding Habits,” Pacific Science (Vol. 60, 2006), 523–530.
- See, respectively, M. Guerrero-Ruiz, et al., “Current Knowledge of Killer Whales in Mexican Pacific,” Fourth International Orca Symposium and Workshop (Centre National de la Recherche ́ Scientifique, France, 2002) 76; J.C. Lopez and D. Lopez, “Killer Whales (Orcinus orca) of Patagonia, and Their Behavior of Intentional Stranding While Hunting Nearshore,” Journal of Mammalogy (Vol. 66, 1985), 181-183; Christophe Guinet et al., “Killer whale predation on bluefin tuna: Exploring the hypothesis of the endurance-exhaustion technique,” Marine Ecology Progress Series (Vol. 347, 2007), 111-119; Christophe Guinet and P. Tixier, “Crozet: Killer Whales in a Remote but Changing Environment,” Journal of the American Cetacean Society (Vol. 40, 2011), 33–38; C.A. Tosh, P.J.N. de Bruyn, and M.N. Bester, “Preliminary Analysis of the Social Structure of Killer Whales, Orcinus orca, at Subantarctic Marion Island,” Marine Mammal Science (Vol. 24, 2008), 929–940; R.R. Reisinger et al., “Prey and seasonal abundance of killer whales at subantarctic Marion Island,” African Journal of Marine Science (Vol. 33, 2011), 99-105; and J.W. Higdon, D.D.W Hauser, and S.H. Ferguson, “Killer Whales (Orcinus orca) in the Canadian Arctic: Distribution, Prey Items, Group Sizes, and Seasonality,” Marine Mammal Science (Vol. 28, 2012), E93–E109.
- See, for instance, Uttam Saikia, Narayan Sharma, and Abhijit Das, “What Is a Species?: An Endless Debate,” Resonance (Vol. 13, 2008), 1049-1064.
- Rüdiger Riesch, “Killer Whales Are Speciating Right in Front of Us,” Scientific American (May 1, 2017).
- In technical biology jargon, this is termed “allopatric speciation.” For a further discussion of how twentieth-century concepts of speciation went beyond Darwin, see Jerry A. Coyne, “Ernst Mayr and the Origin of Species,” Evolution (Vol. 48, No. 1, 1994), 19-30. Coyne writes, “[M]uch of [evolutionary biology] still consists of repeated demonstrations of phenomena first described by Darwin, or of epiphenomena derived from them. The study of speciation, however, is a conspicuous exception. Despite the title of his famous book, Darwin was notably unsuccessful in solving the real problem of organic diversity: why plants and animals in a habitat fall into discrete, nonoverlapping packages. It is widely accepted that his failure came from his inability to conceptualize species as noninterbreeding groups and to recognize that the origin of species was identical to the origin of the barriers to interbreeding. Because Darwin considered species to be only highly evolved morphological varieties (indeed, The Origin of Species should have been called The Origin of Adaptations), he confused adaptation within lineages with the origin of new lineages. Although the two are connected, the former does not automatically produce the latter.” This article is also another useful introduction to the various and quite incompatible species concepts that have plagued debates within biology.
- Population genomic data on killer whales indeed strongly suggests that the (cultural and biological) evolution of ecotypes has been largely sympatric: divergence while maintaining geographic overlap. See Andre E. Moura et al., “Population Genomics of the Killer Whale Indicates Ecotype Evolution in Sympatry Involving Both Selection and Drift,” Molecular Ecology (Vol. 23,No. 21, 2014), 5179−5192; and Andrew D. Foote et al., “Genome-Culture Coevolution Promotes Rapid Divergence of Killer Whale Ecotypes,” Nature Communications (Vol. 7, 2016), Article Number 11693.
- Safina, Becoming Wild (2020), 67.
- ibid 68.
- R.R. Reeves et al., Report of the Workshop on Shortcomings of Cetacean Taxonomy in Relation to Needs of Conservation and Management, April 30–May 2, 2004, La Jolla, California.
- Safina, Becoming Wild (2020), 69.
- See, as a few examples, Robin W. Baird, Peter A. Abrams, and Lawrence M. Dill, “Possible Indirect Interactions Between Transient and Resident Killer Whales: Implications for the Evolution of Foraging Specializations in the Genus Orcinus,” Oecologia (Vol. 89, 1992), 125-132; J.R Boran and S.L. Heimlich, “Social Learning in Cetaceans: Hunting, Hearing and Hierarchies,” in Mammalian Social Learning: Comparative and Ecological Perspectives, eds. Hillary O. Box and Kathleen R. Gibson (Cambridge, Cambridge University Press, 1999), 282-307; Rüdiger Riesch, et al., “Cultural traditions and the evolution of reproductive isolation: ecological speciation in killer whales?,” Biological Journal of the Linnean Society (Vol. 106, No. 1, 2012), 1–17; Rüdiger Riesch, “Killer Whales Are Speciating Right in Front of Us,” Scientific American (May 1, 2017); and Andrew D. Foote et al., “Genome-culture coevolution promotes rapid divergence of killer whale ecotypes,” Nature Communications (Vol. 7:11693, 2016). In the latter, the authors write that killer whales “offer a prime example of how behavioural innovation perpetuated by cultural transmission may have enabled access to novel ecological conditions with altered selection regimes, and thus provide an excellent study system for understanding the interaction between ecological and behavioural variation, and genome-level evolution,” and that “whole-genome resolution confirms that, even in sympatry [i.e. overlapping ranges], contemporary gene flow occurs almost exclusively among individuals of the same ecotype, allowing genomic differentiation to build up between ecotypes so that within an ocean basin ecological variation [passed down culturally] better predicted genetic structuring than geography.” This paper was co-authored by twenty scientists. For examples of scientists discussing culture-driven speciation among very different species, see B.R. Grant and P. R. Grant, 1996. “Cultural inheritance of song and its role in the evolution of Darwin’s finches,” Evolution (Vol. 50, 1996), 2471–87; and P.R. Grant and B. R. Grant, “The secondary contact phase of allopatric speciation in Darwin’s finches,” Proceedings of the National Academy of Sciences of the United States of America (Vol. 106, 2009), 20141–48. In the Grants’ research, socially learned differences in song among Galapagos finches produced reproductive isolation.
- Whitehead and Rendell refer offhandedly to these socially rather than ecologically divergent populations as “sociotypes.” See The Cultural Lives of Whales and Dolphins (2015), 237.
- Graeber and Wengrow, The Dawn of Everything (2021).
- V.B. Deecke, J.K.B. Ford, and P. Spong, “Dialect Change in Resident Killer Whales: Implications for Learning and Cultural Transmission,” Animal Behaviour (Vol. 60, 2000), 629-638.
- This unwritten agreement between the cooperating hunters was known to human whalers as “the Law of Tongue.” See Tom Meade, Killers of Eden: The Killer Whales of Twofold Bay (Dolphin Books, 2002), as well as Luke Rendell, “Lecture 5: Major Study Groups — Whales and Dolphins,” in Animal Cultures: Core Discoveries and New Horizons, online learning series by the Cultural Evolution Society. https://learn.culturalevolutionsociety.org/animal_cultures_module/l05_whales.
- A different perspective on this comes from Belinda Recio. She writes, “The first time the orcas cooperated with them the whalers were surprised, but the local aboriginal peoples were not. In fact, they explained to the whalers that orcas loitering near a baleen whale wasn’t coincidental. The orcas in those coastal Australian waters had been helping the indigenous peoples hunt baleen whales for thousands of years before the Europeans arrived.” So it is possible that this cooperative whale-hunting culture has now been extinguished, but was not in fact short-lived. From Belinda Recio, “Of Orcas and Humans,” Organic Spa Magazine (21 February 2018). https://www.organicspamagazine.com/orcas-and-humans/.
- Starvation is not the immediate cause of death for these killer whales. Being at the highest trophic level of marine food chains, killer whales (of all ecotypes but particularly those who prey upon mammals) have bioaccumulated huge concentrations of deadly toxins from human industrial activities in their blubber. When they face food stresses, their bodies consume their blubber for energy, bringing these pollutants flooding back into their bodies and killing them with toxic shock. It is the metabolic interplay between these distinct human violences that kills them.
- Despite numerous observed encounters between sea otters and killer whales over decades, no killer whale had been observed preying upon a sea otter before 1991. “Killer Whales Have Begun Preying On Sea Otters, Causing Disruption Of Coastal Ecosystems In Western Alaska,” Science Daily (16 October 1998), https://www.sciencedaily.com/releases/1998/10/981016075816.htm. See also J.A. Estes, et al., “Causes and Consequences of Marine Mammal Population Declines in Southwest Alaska: A Food Web Perspective,” Philosophical Transactions of the Royal Society B (Vol. 364, No. 1524, 2009), 1647–1658.
- Whitehead and Rendell, The Cultural Lives of Whales and Dolphins (2015), 133.
- As with humpback lobtail feeding behavior, this spread best fit to a social science-derived Wave of Advance mathematical model. See Zachary A. Schakner et al, “Using models of social transmission to examine the spread of longline depredation behavior among sperm whales in the Gulf of Alaska,” PLoS One (Vol. 9, No. 10, 2014); and Michael F. Sigler, et al., “Sperm whale depredation of sablefish longline gear in the northeast Pacific Ocean,” Marine Mammal Science (Vol. 24, No. 1, 2008), 16– 27.
- Whitehead and Rendell, Cultural Lives of Whales and Dolphins (2015), 147. Males also tend to have a more generalist diet, feeding upon fish and crustaceans as well as squid (P.B. Best, “Food and feeding of sperm whales Physeter macrocephalus off the west coast of South Africa,” South African Journal of Marine Science (Vol. 21, No. 1, 1999), 393–413; Sónia Mendes et al., “Stable carbon and nitrogen isotope ratio profiling of sperm whale teeth reveals ontogenetic movements and trophic ecology,” Oecologia (Vol. 151, No. 4, 2007), 605–615). For all these reasons, early accounts of sperm whales assumed that males and females were different species. Whalers also somehow reached the perfectly wrong conclusion that they were a harem species, with each family unit imagined to be led by a dominant male.
- Although false killer whales and some sharks occasionally prey upon sperm whale calves, the primary nonhuman threat sperm whales face are killer whales—at least, those handful of killer whale communities who have a cultural affinity for hunting them.
- Safina, Becoming Wild (2020), 15.
- Whitehead and Rendell, The Cultural Lives of Whales and Dolphins (2015), 63-64.
- Elin Kelsey, Watching Giants, 67; Francois Sarano, “Kin relationships in cultural species of the marine realm: case study of a matrilineal social group of sperm whales off Mauritius island, Indian Ocean,” Royal Society Open Science (Vol. 8, No. 2, 2021). https://royalsocietypublishing.org/doi/10.1098/rsos.201794#d1e1340. The latter source documents a unit where the vast majority of a calf’s nursing sightings were with someone other than their biological mother.
- Safina, Becoming Wild, 15.
- Craig Welch, “The Hidden World of Animal Culture,” National Geographic (April 15, 2021). https://www.nationalgeographic.com/magazine/article/the-hidden-world-of-whale-culture-feature.
- Safina, Becoming Wild, 16. This is most common among sperm whales in the Pacific.
- Hal Whitehead, “Consensus Movements by Groups of Sperm Whales,” Marine Mammal Science (Vol. 32, No. 4, 2016), 1402-1415.
- This so-called “spermaceti organ” in front of the skull is the source of the sperm whale’s unfortunate name: whalers who hacked them open for their valued oils thought that those in their sonar system looked like semen. Whitehead and Rendell, Cultural Lives of Whales and Dolphins, 60-61. See also B. Møhl, et al., “Sperm whale clicks: directionality and source level revisited,” Journal of the Acoustical Society of America (Vol. 107, 2000), 638–48.
- Luke Rendell and Hal Whitehead, “Vocal Clans in Sperm Whales (Physeter Macrocephalus),” Proceedings of the Royal Society of London, Series B (Vol. 270, No. 1520, 2003), 225-231. Codas are clearly for social rather than echolocative purposes because interacting individuals overlap their clicking in “duet-like sequences,” and they are produced at high rates at the surface but not during their solitary dives for food. See Tyler M. Schulz et al., “Overlapping and Matching of Codas in Vocal Interactions Between Sperm Whales: Insights into Communication Function,” Animal Behavior (Vol. 76, 2008), 1977-1988; and Hal Whitehead and Linda Weilgart, “Patterns of Visually Observable Behavior and Vocalizations in Groups of Female Sperm Whales,” Behaviour (Vol. 118, 1991), 275–296.
- Safina, Becoming Wild, 19.
- Safina, Becoming Wild, 30.
- Taylor A. Hersch et al., “Evidence from sperm whale clans of symbolic marking in non-human cultures,” PNAS (Vol. 119, No. 37, 2022).
- Shane Gero et al., “Socially segregated, sympatric sperm whale clans in the Atlantic Ocean,” Royal Society Open Science (Vol. 3, No. 6, 2016); and Thiago Orion Simões Amorim et al., “Coda Repertoire and Vocal Clans of Sperm Whales in the Western Atlantic Ocean,” Deep Sea Research Part I: Oceanographic Research Papers (Vol. 160, 2020).
- Léonie A.E. Huijser et al., “Vocal repertoires and insights into social structure of sperm whales (Physeter macrocephalus) in Mauritius, southwestern Indian Ocean,” Marine Mammal Science (Vol. 36, No. 2, 2020), 638-657.
- Hal Whitehead and Luke Rendell, “Movements, habitat use and feeding success of cultural clans of South Pacific sperm whales,” Journal of Animal Ecology (Vol. 73, No. 1, 2004), 190-196.
- Whitehead and Rendell, The Cultural Lives of Whales and Dolphins, 153.
- Hal Whitehead, “Variation in the visually observable behavior of groups of Galápagos sperm whales,” Marine Mammal Science (Vol. 15, No. 4, 1999), 1181–97; Whitehead and Rendell, “Movements, habitat use and feeding success of cultural clans of South Pacific sperm whales” (2004).
- Marianne Marcoux, Luke Rendell, and Hal Whitehead, “Indications of fitness differences among vocal clans of sperm whales,” Behavioural Ecology and Sociobiology (Vol. 61, 2007), 1093–98.
- Whitehead and Rendell, The Cultural Lives of Whales and Dolphins, 153.
- Safina, Becoming Wild, 62-63; Hal Whitehead, Sperm Whales: Social Evolution in the Ocean (Chicago, Chicago University Press, 2003), 300; T. Lyrholm et al., “Sex-biased dispersal in sperm whales: contrasting mitochondrial and nuclear genetic structure of global populations,” Proceedings of the Royal Society of London, ser. B (Vol. 266, 1999), 347–54.
- Whitehead and Rendell, The Cultural Lives of Whales and Dolphins, 153-154.
- Hal Whitehead refers to this as “cultural hitchhiking”: mitochondrial genetic markers are proliferated not because of their own contribution to biological fitness but because they are tied to a certain set of successful cultural traits, the primary determinants of inter-group divergence in reproductive success. See his TedX talk on whale culture: https://www.youtube.com/watch?v=9uyGXoMaXns.
- Hal Whitehead, et al., “Non-geographically based population structure of South Pacific sperm whales: dialects, fluke-markings and genetics,” Journal of Animal Ecology (Vol. 67, No. 2, 1998), 253-262.
- Luke Rendell et al., “Can genetic differences explain vocal dialect variation in sperm whales, Physeter macrocephalus?,” Behavioral Genetics (Vol. 42, 2012), 332–43; Jenny Christal, Hal Whitehead, and Erland Letteval, “Sperm Whale Social Units: Variation and Change,” Canadian Journal of Zoology (Vol. 76, 1998), 1431-1440.
- Personal correspondence with Hal Whitehead (January 31, 2024), Luke Rendell (February 2, 2024), and Shane Gero (February 25, 2024).
- Hal Whitehead, et al., “Multilevel societies of female sperm whales (Physeter macrocephalus) in the Atlantic and Pacific: why are they so different?,” International Journal of Primatology (Vol. 33, 2012), 1142–64.
- Shane Gero et al., “Socially segregated, sympatric sperm whale clans in the Atlantic Ocean,” Royal Society Open Science (Vol. 3, No. 6, 2016).
- Safina, Becoming Wild, 50-52.
- Robert L. Pitman, et al., “Killer Whale Predation on Sperm Whales: Observations and Implications,” Marine Mammal Science (Vol. 17, 2001), 494-507. This paper also includes an account of a desperately failed attempt at defense from killer whales by a sperm whale unit that seemed unknowledgeable about how to protect themselves, sustaining terrible damage in vain efforts to protect each of their members—possibly fatal for the entire unit. The researchers discussed it as an instance of altruistic behavior having occasional disastrous consequences, despite its usual adaptiveness in sperm whale life.
- Hal Whitehead, Tim D. Smith, and Luke Rendell, “Adaptation of Sperm Whales to Open-boat Whalers: Rapid Social Learning on a Large Scale?,” Biology Letters (Vol. 17, No. 3, 2021). These strategies included fleeing upwind of the ship and diving, but most importantly entailed abandoning their defensive response to killer whales (collective encirclement), their only natural predator, which only served to make themselves more vulnerable to the human whalers.
- Herman Melville, Moby-Dick: Or, the Whale (New York, Bantam Classics, 1981), Chapter 87.
- Although Whitehead and Rendell were the first to suggest the term “clan” to describe the social units structured by vocal dialects that they discovered (a nod to the clans of killer whales in the north Pacific), they too have pointed to the possibility that the (ethnographically loaded) term “nation” might in fact be more appropriate. They write, “We suspect there is something different about sperm whale clans; they are more than just a level of social structure, they are a cultural identity. So just as a modern human has a vital ethnicity, usually shared with millions, on top of his or her ‘large tribe’ of a few thousand—that might be equivalent to a village, neighborhood, or high school—a female sperm whale in the Pacific has her clan. This leads to ‘us’—my clan, or nation—and ‘them’—members of other clans or nations.” (From The Cultural Lives of Dolphins and Whales, 156.)
- 1986 was the year the International Whaling Commission’s moratorium on commercial whaling came into effect, ending most whaling worldwide.
- Whitehead, et al., “Multilevel societies of female sperm whales (Physeter macrocephalus) in the Atlantic and Pacific: why are they so different?,” 2012. They write, “the fundamental distinction between the oceans is in the culturally driven behavior of killer whales, for which sperm whales are on the menu in the Pacific, but not in the Atlantic. This, apparently arbitrary, difference in the cultures of killer whales in the two oceans then may have had a large role in shaping the multilevel social systems of the sperms.”
- Whitehead and Rendell, The Cultural Lives of Dolphins and Whales, 155.
- Safina, Becoming Wild, 81-84.
- Mauricio Cantor et al., “Cultural Turnover Among Galapagos Sperm Whales,” Royal Society Open Science (Vol.3, No. 10, 2016).
- Luke Rendell, “Lecture 5: Major Study Groups – Whales and Dolphins,” Animal Cultures: Core Discoveries and New Horizons (The Cultural Evolution Society’s Online Learning Series). https://learn.culturalevolutionsociety.org/animal_cultures_module/l05_whales. In a different talk entitled “The Minds of Whales,” he similarly stated that “It’s a really interesting study that makes you question whether you’re studying biology or history.”
- Bookchin, Remaking Society, 21.
- Toshio Kasuya, “Cetacean Biology and Conservation: A Japanese Scientist’s Perspective Spanning 46 Years” (the Kenneth S. Norris Lifetime Achievement Award Lecture, presented 29 November 2007 in Cape Town, South Africa), Marine Mammal Science (Vol. 24, No. 4, 2008), 749-773.
- Philippa Brakes, et al., “Animal Cultures Matter for Conservation,” Science (Vol. 363, No. 6431, 2019).
- Karen McComb et al., “Matriarchs as Repositories of Social Knowledge in African Elephants”, Science (Vol. 292, No. 5516, 2001), 491-494 (494).
- Safina, Becoming Wild. This theory is also developed by Cristine H. Legare and Mark Nielsen in “Imitation and Innovation: The Dual Engines of Cultural Learning,” Trends in Cognitive Sciences (Vol. 19, No. 11, 2015), 688-699, with a narrower focus on human children.
- Jane Goodall, “Do Chimpanzees Have Souls?,” in: Spiritual Information: 100 Perspectives on Science and Religion, ed. C.L. Harper (West Conshohocken, PA, Templeton Press, 2005), 275-278.
- Murray Bookchin, Urbanization Without Cities: The Rise and Decline of Citizenship (Montreal and New York, Black Rose Books, 1992), xvii-xviii.
